Instead, they are Diastereomers (two structures that differ at at least 1 chiral center and are not mirror images).]T or F Alpha-D-Glucose and Beta-D-Glucose are Epimers [True. Alpha and Beta Glucose only differ at the anomeric Carbon (Carbon 1). Epimers are two structures that differ at only1 chiral center.
What is the difference between alpha and beta glucose isomers?
Alpha and beta are both glucose isomers, but they differ only in the position of their -OH (hydroxyl) and -H (hydrogen) groups on carbon 1. Beta glucose has its -OH group above the ring. On the other hand, Alpha glucose has its -OH attached below the ring.
Is alpha (D+) glucose the same as beta (D-) glucose?
Is alpha (D+) glucose same as beta (D-) glucose? No. In the name D (+) Glucose, D represents the orientation of the hydroxyl group at the chiral carbon that is farthest from the highest oxidised carbon (Aldehyde group in this case) with respect to glyceraldehyde.
How many isomers of glucose are there?
Glucose has two isomers (types), the alpha and the beta. When it comes to alpha vs. beta glucose, what are the differences and similarities between the two?
What do alpha and beta prefixes mean in chemistry?
Chemists use prefixes like alpha and beta to denote different versions of glucose and other sugar molecules. To the uninitiated, these prefixes may seem mysterious, but once you understand sugar structure, their nature and purpose will become clearer. Each molecule of glucose has a carbon backbone with -OH groups and hydrogen atoms attached to it.
See more

Why are alpha and beta glucose diastereomers?
The $\alpha - D - $glucose and $\beta - D - $glucose are anomers as they differ at carbon 1 only. D. The epimers are diastereomers that contain more than one chiral centre but differ from each other in the absolute configuration at only one chiral centre.
What type of isomers are alpha and beta glucose?
These two forms of glucose are (stereo)isomers, because they contain the same atoms, but they differ in the arrangement of their atoms in space. They can also be called epimers because they represent different configurations of atoms about a single stereogenic centre - in this case carbon 1.
What is the relationship between alpha and beta glucose?
The glucose has two isomers, alpha glucose and beta glucose. The main difference between the two lies in the orientation of the (-OH) hydroxyl group. In both cases, the hydroxyl group gets connected to the first carbon atom, just the geometry is different.
Are alpha and beta anomers diastereomers?
Anomer – the name given to two diastereomeric monosaccharides that are epimers at the anomeric carbon. The two anomers are described with the terms α (“alpha”) and β (“beta”), defined above.
Are anomers diastereomers?
Because anomers are diastereomers of each other, they often differ in physical and chemical properties. One of the most important physical properties that is used to study anomers is the specific rotation, which can be monitored by polarimetry.
Which word best describes the relationship between α glucose and β glucose?
The compounds α−D glucose and β−D glucose are not mirror images of each other and are hence not enantiomers. They can be considered to be diastereomers as they are not mirror images of each other. They are also considered as anomers as they differ in only C-1 configuration. Hence the option D is correct.
What's the difference between alpha and beta glucose and why is it important in living systems?
The key difference between alpha (α) and beta (β) glucose is the orientation of hydroxyl (-OH) group attached to the first carbon atom. The two dimensional graphical representation of these isomeric glucose structures shows that the α-glucose has (1-hydroxyl) and (4-hydroxyl) orientations on the same side.
What is the structural difference between alpha and beta glucose?
They differ only in the direction that -H and -OH groups point on carbon 1 (See the jmol images below). When alpha-glucose molecules are joined chemically to form a polymer starch is formed. When beta-glucose molecules are joined to form a polymer cellulose is formed.
What is the difference between alpha and beta glucose a level biology?
The main difference between alpha and beta glucose is that the –OH group attached to the first carbon atom in alpha glucose is located on the same side as the –CH2OH group whereas the –OH group attached to the first carbon atom of in beta glucose is located on the opposite side from the –CH2OH group.
Are epimers diastereomers?
Epimer: One of a pair of stereoisomers that differ in the absolute configuration of a single stereocenter. When the molecule has only one stereocenter then the epimers are enantiomers. When the molecule has two or more stereocenters then the epimers are diastereomers.
What does diastereomer mean?
Stereoisomers that are not related as an object and its mirror image are called diastereomers; Diastereomers are stereoisomers that are not mirror images.
Are anomers enantiomers?
So in order for the anomers to be enantiomers, the compound actually must have zero stereocenters in the open form. If you consider glycoaldehyde a carbohydrate and you consider the three-membered ring cyclic hemiacetal a reasonable structure, then you have found a set of anomers that are enantiomers.
Are alpha and beta glucose optical isomers?
α-D-glucose and β-D-glucose are stereoisomers - they differ in the 3-dimensional configuration of atoms/groups at one or more positions. Note that the structures are almost identical, except that in the α form, the OH group on the far right is down, and, in the β form, the OH group on the far right is up.
Are alpha and beta sugars isomers?
The cyclic forms of carbohydrates can exist in two forms, α- and β- based on the position of the substituent at the anomeric center. The two forms are sometimes described as "anomers" since they are isomers at the anomeric center.
What is the structure of alpha and beta glucose?
Alpha Glucose: Alpha glucose is an isomer of D-glucose that has the –OH group of the first carbon atom positioned on the same side as the –CH2OH group. Beta Glucose: Beta Glucose is an isomer of D-Glucose that has the –OH group of the first carbon atom positioned on the opposite side from the –CH2OH group.
What are isomers of glucose?
Glucose and its isomers Fructose is a structural isomer of glucose and galactose, meaning that its atoms are actually bonded together in a different order. Glucose and galactose are stereoisomers (have atoms bonded together in the same order, but differently arranged in space).
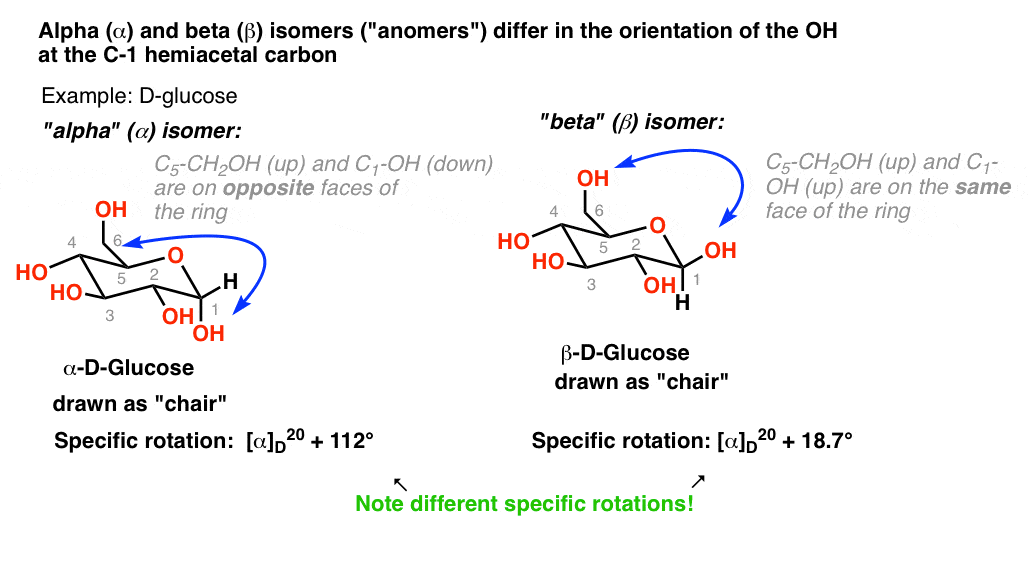
How many isomers are there in D-glucose?
In our recent post on ring-chain tautomerism , we said that there are two isomers of D-glucose in its 6-membered ring (pyranose) form. These two diastereomers which, to make matters more confusing, are called anomers in the context of sugar chemistry differ in the orientation of the hydroxyl group on C-1. (Note that C-1 is a hemiacetal . ) In the alpha () anomer, the OH group on C-1 is on theopposite side of the ring as the chain on C-5. In the beta () anomer, the OH group on C-1 is on the sameside of the ring as the C-5 substituent. Each of these two forms can be synthesized and isolated as pure compounds. The alpha () anomer of D-glucose has a specific rotation of +112 degrees in water. The beta () anomer of D-glucose has a specific rotation of +19 degrees. (18.7 actually, but rounding up to 19). Heres the interesting thing.When either anomer is dissolved in water, the value of the specific rotation changes over time, eventually reaching the same value of +52.5. The specific rotation of -D-glucopyranose decreases from +112 to +52.5. The specific rotation of-D-glucopyranose increases from +19 to +52.5. This behaviour is calledmutarotation (literally, change in rotation). Hold on. Isnt specific rotation of a molecule supposed to remain the same? Yes if it is indeed the same molecule! And therein lies the answer to the puzzle. For when the solutions whose specific rotations have changed to +52.5 are analyzed, they are found to no longer consist of 100% alpha () or 100% beta ()anomers, but instead a ratio of alpha () (36%) and beta () (64% ) isomers. Wait. What happened here? How did the alpha convert to the beta, and vice-versa? You may recall how we said in the last post on ring-chain tautomerism that the cyclic hemiacetal forms of sugars are in equilibrium with the str Continue reading >>
What happens when glucose is dissolved in water?
When dissolved in water, these glucose tablets will yield a mixture of alpha and beta glucose.Photo Credit: YakubovAlim/iStock/Getty Images Based in San Diego, John Brennan has been writing about science and the environment since 2006. His articles have appeared in "Plenty," "San Diego Reader," "Santa Barbara Independent" and "East Bay Monthly." Brennan holds a Bachelor of Science in biology from the University of California, San Diego. Glucose is one of the "simple sugars" -- an ironic name, because the chemistry of these compounds is rather complex. The naming system for sugars reflects this complexity. Chemists use prefixes like alpha and beta to denote different versions of glucose and other sugar molecules. To the uninitiated, these prefixes may seem mysterious, but once you understand sugar structure, their nature and purpose will become clearer. Each molecule of glucose has a carbon backbone with -OH groups and hydrogen atoms attached to it. At the top of the chain, an oxygen atom is double-bonded to a carbon atom; collectively, these two atoms are called a carbonyl group. The carbon backbone of the glucose molecule can coil up so that an -OH group near the bottom end of the chain attacks the carbonyl carbon and the glucose molecule forms a ring. This ring-shaped structure is the cyclic form of glucose, while the straight chain structure is the linear form. In solution, the cyclic form is by far the more common. Glucose can form either five-membered or six-membered rings. The six-membered ring is much more common, and in solution the vast majority of glucose molecules are found to have six-membered rings. Since linear and cyclic forms can inter-convert, however, no glucose molecule is ever fixed in the six-membered ring form; it can go back and forth. It does sp Continue reading >>
What is the anomeric centre of a sugar?
If you would like to suggest an addition or correction, please contact the page's Responsible Curator directly by e-mail, or using this form . The anomeric centre of a sugar is a stereocentre created from the intramolecular formation of an acetal (or ketal) of a sugar hydroxyl group and an aldehyde (or ketone) group. The two stereoisomers formed from the two possible stereochemistries at the anomeric centre are called anomers. They are diastereoisomers of one another. The configuration at the anomeric centre (that derived from the carbonyl carbon) is denoted alpha- (-) or beta- (-) by reference to the stereocentre that determines the absolute configuration. In a Fischer projection, if the substituent off the anomeric centre is on the same side as the oxygen of the configurational (D- or L-) carbon, then it is the --anomer. If it is directed in the opposite direction it is the -anomer. Example 1. Fischer projections and Haworth conformational projections of L-arabinose. Example 2. Fischer projections and Haworth conformational projections of D-fructose. In the case of D-hexopyranoses drawn in the 'usual' Haworth projection, the -D-anomer is the isomer with the anomeric substituent on the opposite face to the C5 (hydroxymethyl) substitutent, ie directed down; the -D-anomer is that with the anomeric substituent being on the same face as the C5 hydroxymethyl substitutent, ie directed up. For L-hexoses the -L-anomer has the anomeric group pointing up; the -L-anomer has this group pointing down. Example 3. Fischer projections and Haworth conformational projections of D-glucose. Continue reading >>
Is Alpha (d+) Glucose Same As Beta (d-) Glucose?
Is alpha (D+) glucose same as beta (D-) glucose? No. In the name D (+) Glucose, D represents the orientation of the hydroxyl group at the chiral carbon that is farthest from the highest oxidised carbon (Aldehyde group in this case) with respect to glyceraldehyde. D says that the hydroxyl group is on the right side (In fischer projection). L says the opposite. Where as (+) and (-) represent the direction of rotation of plane polarised light {Optical rotation} (Determined experimentally) by the solution as a whole. When a water molecule adds to the glucose molecule, the aldehyde group turns into hemiacetal (Ring is formed between 1st and 5th carbon), making the first carbon (previously aldehyde) chiral. Alpha and Beta represent the orientation of hydroxide group (R and S) at that new chiral carbon (Anomeric carbon). Hence those forms are called anomers. Pure Alpha glucose has a positive optical rotation and the Beta form has the opposite rotation (but of different magnitude). The compounds which you have mentioned in the question are pure alpha and pure beta forms of glucose. If you get some random D glucose from somewhere and you some how determined that it has a positive optical rotation,it doesnt mean that it is alpha D glucose. It may also contain beta D glucose each cancelling the effect of other and ultimately resulting a positive optical rotation (Overall dominated by alpha D glucose). Continue reading >>
Why are diastereomers different?
Due to their different shape, diastereomers can have different physical and chemical properties. This is perhaps especially true of diastereomers involved in biological systems. According to IUPAC the term "geometric isomerism" is an obsolete synonym of "cis-trans isomerism" and its use is strongly discouraged.
What Is The Difference Between Enantiomers & Diastereomers?
What is the Difference Between Enantiomers & Diastereomers? Watch short & fun videos Start Your Free Trial Today Laura has a Masters of Science in Food Science and Human Nutrition and has taught college Science. Log in or sign up to add this lesson to a Custom Course. Custom Courses are courses that you create from Study.com lessons. Use them just like other courses to track progress, access quizzes and exams, and share content. Organize and share selected lessons with your class. Make planning easier by creating your own custom course. Create a new course from any lesson page or your dashboard. Click "Add to" located below the video player and follow the prompts to name your course and save your lesson. Click on the "Custom Courses" tab, then click "Create course". Next, go to any lesson page and begin adding lessons. Edit your Custom Course directly from your dashboard. Name your Custom Course and add an optional description or learning objective. Create chapters to group lesson within your course. Remove and reorder chapters and lessons at any time. Share your Custom Course or assign lessons and chapters. Share or assign lessons and chapters by clicking the "Teacher" tab on the lesson or chapter page you want to assign. Students' quiz scores and video views will be trackable in your "Teacher" tab. You can share your Custom Course by copying and pasting the course URL. Only Study.com members will be able to access the entire course. Enantiomers and diastereomers are types of stereoisomers. In this lesson we will learn what the difference is between these types of stereoisomers and how to differentiate between them. Take a look at your hands - they are non-superimposable mirror images of each other. This means that they are mirror images of each other, but you can't s Continue reading >>
What is the simplest form of carbohydrates?
Structural Biochemistry/Carbohydrates/Monosaccharides Monosaccharides are the simplest form of carbohydrates and may be subcategorized as aldoses or ketoses . The sugar is an aldose if it contains an aldehyde functional group. A ketose signifies that the sugar contains a ketone functional group. Monosaccharides may be further classified based on the number of carbon atoms in the backbone, which can be designated with the prefixes tri- (3), tetr- (4), pent- (5), hex- (6), hept- (7), etc. in the name of the sugar. Monosaccharides are often represented by a Fischer Projection, a shorthand notation particularly useful for showing stereochemistry in straight chained organic compounds. The L and D confirmations represent the absolute configuration of the asymmetric carbon farthest away from the ketone or aldehyde group on the monosaccharide. On the Fischer projection, if the farthest hydroxyl (-OH) group is on the right, then it is classified as D sugar, if the hydroxyl group is on the left, then it is a L sugar. Enantiomers, Diastereoisomers (anomerism), and Epimers Example of Diastereomers. The areas marked blue indicate the differing stereogenic centers. Example of an Enantiomer. The blue indicates the D-isomer and the red indicates the L-isomer Due to the fact that carbohydrates contain multiple stereocenters, many isomers are possible including enantiomers, diastereoisomers, and epimers. Two carbohydrates are said to be enantiomers if they are nonsuperimposable mirror images of one another. An example of an enantiomer is the D and L isomers of glucose, as shown by the figure to the right. A second type of isomer seen in carbohydrates are diastereoisomers. Carbohydrates are classified as diastereomers if their chiral carbons are connected to the exactly the same substra Continue reading >>
What are stereoisomers that are not mirror images of each other?
Hence different optical isomers may have varient biological effects. Diastereomers are stereoisomers that are not mirror images of each other that is they are not linked with reflection operation unlike of enantiomers. They posses same physical properties. One of the example include meso compounds.
What is the term for stereoisomers that are not optical isomers?
The exact term for stereoisomers that are not optical isomers is diastereomers. A special kind of diastereomer is an epimer. Epimers are diastereomers that differ at one of several asymmetric carbon atoms. There is also something called an anomer, a special type of epimer.
How does each stereocenter affect the number of stereoisomers?
Each stereocenter gives rise to two different configurations and thus increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. [3] .
What is a diastereoisomer?
Diastereomer - Wikipedia. Diastereomers (sometimes called diastereoisomers) are a type of a stereoisomer . [1] . Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. [2] .
