
What is heat in science for kids?
Science >> Physics for Kids. Heat is the transfer of energy from a one object to another due to a difference in temperature. Heat can be measured in joules, BTUs (British thermal unit), or calories. Heat and temperature are closely related, but they are not the same thing.
Do you know the difference between heat and temperature?
But do we know the difference between heat and temperature? It is important to realize the minute difference between heat and temperature scales of measuring hotness or coldness of any object. They are interconnected, but contrary to popular belief, they do not mean the same thing.
What is heat and how is It measured?
Heat is the transfer of energy from a one object to another due to a difference in temperature. Heat can be measured in joules, BTUs (British thermal unit), or calories.
Is heat a physical property of an object?
It is a measurable physical property of an object—also known as a state variable. Other measurable physical properties include velocity, mass, and density, to name a few. Heat is a transfer of thermal energy caused by a difference in temperature between molecules.
What is the difference between heat and temperature?
Heat is the total energy of the motion of the molecules of a substance, whereas the temperature refers to the measure of the average energy of the motions of the molecules in the substance. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc.
Why are heat and temperature mixed up?
The reason why the concept of heat and temperature might be mixed up is because of how closely they are related in real life. If you add heat to something, its temperature goes up. If you reduce temperature, you are taking its heat away. Let’s look at what they actually represent.
What happens when you heat a substance?
There are two things that can happen when you heat a substance; The substances undergo a rise in temperature corresponding to the increase in the kinetic energy of the molecules; i.e. the heat gives the molecules extra energy to move around with more speed, and this increase in speed implies an increase in temperature.
What happens when you double the temperature of a substance?
As we said earlier, the temperature is directly related to the kinetic energy of the molecules, therefore if you double the temperature (in degrees Kelvin) of a substance, you double the average kinetic energy possessed by those molecules.
What is the measure of the thermal energy of a substance?
Temperature is the measure of the thermal energy or average heat of the molecules in a substance. It’s a measure of the number of atoms multiplied by the energy possessed by each atom. It is like a level which determines the direction in which the heat will flow.
What is the unit of temperature?
The SI Unit of temperature is degrees, Kelvin.
Is heat the same as temperature?
They are interconnected, but contrary to popular belief, they do not mean the same thing. Knowing the distinction between heat and temperature can lead to a clearer understanding of the world around us. In this article, we will define both heat and temperature and reach an understanding of how they are related, but not identical.
What does it mean when you think of heat and temperature?
When you think of heat and temperature, your brain might instantly go to how hot something is. For example, the heat of the burner on the stove. Therefore, it's easy to get these two terms confused. But now you know better.
What Is Heat?
The heat energy the burner creates to warm up the water is called heat. By definition, heat is a form of energy where energy moves from a hot area to a colder area. In science, you might also hear the definition of heat as the total kinetic energy of an object or particle. While scientists like to get all sciency in their explanations, they are basically talking about how heat moves.
How to know if a turkey is cooked to the right temperature?
If you need to make sure that your roasting turkey has reached the right temperature for eating, you stick a thermometer in it. The thermometer will measure the heat inside the turkey to the exact degree. This is pretty much the same technique a meteorologist or physicist might use, but their thermometers are specialized to their specific fields.
What is the tool used to measure heat in a chemical reaction?
This makes sense, since the tool used to measure heat in a chemical reaction is called a calorimeter .
What is the definition of heat?
In science, you might also hear the definition of heat as the total kinetic energy of an object or particle. While scientists like to get all sciency in their explanations, they are basically talking about how heat moves.
What does temperature mean?
When you think of temperature, you might think of the evening weather. When the meteorologist tells you how cold it is outside, they are talking about temperature. Therefore, it should come as no surprise that temperature measures how cold or hot something is. This could be the air outside or even the temperature of your boiling water. Scientists will also define temperature as the average kinetic energy of a substance. Let’s see this in action.
How many different ways does heat transfer?
In total, there are three different ways heat energy can transfer, called conduction, convection, and radiation. If you’ve used a stove, convection oven, and microwave to cook food, you’ve used all three types.
What is the difference between heat and temperature?
The core difference is that heat deals with thermal energy, whereas temperature is more concerned with molecular kinetic energy .
What is the temperature of a substance?
Temperature describes the average kinetic energy of molecules within a material or system and is measured in Celsius (°C), Kelvin (K), Fahren heit (° F), or Rankine (R). It is a measurable physical property of an object—also known as a state variable. Other measurable physical properties include velocity, mass, and density, to name a few.
What is the transfer of thermal energy caused by a difference in temperature between molecules?
Heat is a transfer of thermal energy caused by a difference in temperature between molecules.
Does ice gain thermal energy?
This is known because temperature is in fact the measure of the average kinetic energy of the molecules. Furthermore, the ice will continue to gain thermal energy causing its molecules to move faster and eventually break their intermolecular bonds or melt.
Is heat a property or a property?
Heat is the transfer of thermal energy, whereas temperature is a property the object exhibits.
Is heat a process variable?
An object can gain heat or lose heat, but it cannot have heat. Heat is a measure of change, never a property possessed by an object or system. Therefore, it is classified as a process variable .
Does thermal energy change the temperature of a substance?
In conclusion, the transfer of heat or thermal energy will typically change the temperature of the substance, but not always! For example, at the moment when the ice in the bowl turns to water those water molecules will be at the exact same temperature as when they were ice. In this case, instead of the thermal energy doing work to increase the kinetic energy, it does work to break the intermolecular bonds, causing a change of state. However, as time goes on the temperature of the recently melted ice will increase until everything within the bowl reaches equilibrium—meaning a consistent temperature throughout.
What is heat in science?
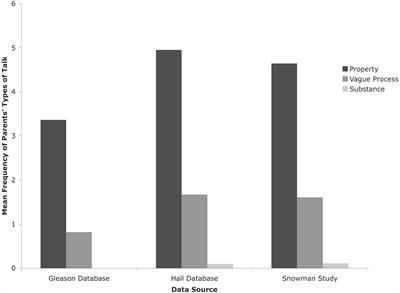
Heat is a substance that flows in and out of objects. Interestingly, research on children’s thinking shows that many children have the same “caloric” view of heat that many eighteenth-century scientists (like Lavoisier) had.
What does it mean when something is hot?
4. When something is hot or warm, there are more atoms in it, making it heavier. Probably from their experiences children often have the impression that the hotter something is, the more “stuff” it has — for example, they might have observed that a room full of people generate a lot of heat.
What does it mean to be cold?
Simply put, cold is the feeling that we get when heat is transferred away from our skin. 3. Heat and temperature are the same thing. In elementary grades, many children have not yet fully grasped the abstract concepts of energy and particles.
Why do children think of cold as a property?
Researchers have also shown that children sometimes think of cold as a property of a certain substance, perhaps because certain materials, including metals, always feel cold to the touch, even at room temperature. Simply put, cold is the feeling that we get when heat is transferred away from our skin.
Does adding heat to matter increase volume?
While adding heat to matter increases its volume, the principle of conservation of mass states that the amount of matter remains constant. Hence, as Lydia discovers in the video, when heat was added to the thermometer, the volume and density (mass/volume) of the red liquid changed, but the weight stayed the same. 5.
Is temperature a measure of motion?
As we’ve said elsewhere, scientific language is more precise than everyday language, which often blurs the distinction between heat and temperature. As stated in the video, heat is simply a transfer of energy from faster-moving particles to slower-moving ones; temperature is a measure of the average motion of particles. 4.
Can children measure temperature?
Children don’t often have direct experience with measuring the temperature in these two situations. No matter how much ice is added to ice water, it can’t get any colder than freezing and, when water reaches its boiling point, it turns into water vapor, leaving the remaining water at a constant temperature.
How are heat and temperature related?
Note that they have different units: temperature typically has units of degrees Celsius () or Kelvin ( ), and heat has units of energy, Joules ( ). Temperature is a measure of the average kinetic energy of the atoms or molecules in the system. The water molecules in a cup of hot coffee have a higher average kinetic energy than the water molecules in a cup of iced tea, which also means they are moving at a higher velocity. Temperature is also an intensive property, which means that the temperature doesn't change no matter how much of a substance you have (as long as it is all at the same temperature!). This is why chemists can use the melting point to help identify a pure substance the temperature at which it melts is a property of the substance with no dependence on the mass of a sample.
How do we measure heat?
How can we measure heat? Here are some things we know about heat so far: 1 When a system absorbs or loses heat, the average kinetic energy of the molecules will change. Thus, heat transfer results in a change in the system's temperature as long as the system is not undergoing a phase change. 2 The change in temperature resulting from heat transferred to or from a system depends on how many molecules are in the system.
What is the zeroth law of thermodynamics?
The zeroth law of thermodynamics says that no heat is transferred between two objects in thermal equilibrium; therefore, they are the same temperature.
How does kinetic energy transfer between two systems?
When the two systems are in contact, heat will be transferred through molecular collisions from the hotter system to the cooler system . The thermal energy will flow in that direction until the two objects are at the same temperature. When the two systems in contact are at the same temperature, we say they are in thermal equilibrium.
How does heat capacity work?
The heat capacity tells us how much energy is needed to change the temperature of a given substance assuming that no phase changes are occurring . There are two main ways that heat capacity is reported. The specific heat capacity (also called specific heat), represented by the symbol or , is how much energy is needed to increase the temperature of one gram of a substance by or . Specific heat capacity usually has units of . The molar heat capacity, or , measures the amount of thermal energy it takes to raise the temperature of one mole of a substance by or , and it usually has units of . For example, the heat capacity of lead might be given as the specific heat capacity, , or the molar heat capacity, .
Why is heat considered a process quantity?
Heat is sometimes called a process quantity, because it is defined in the context of a process by which energy can be transferred. We don't talk about a cup of coffee containing heat, but we can talk about the heat transferred from the cup of hot coffee to your hand. Heat is also an extensive property, so the change in temperature resulting from heat transferred to a system depends on how many molecules are in the system.
What is thermal energy transferred from a hotter system to a cooler system that are in contact?
Heat, , is thermal energy transferred from a hotter system to a cooler system that are in contact.
How is the temperature of an object determined?
The temperature of an object is determined by how fast its molecules are moving. The faster the molecules are moving the higher the temperature. We say objects that have a high temperature are hot and objects with a low temperature are cold. Transferring of Heat.
How does heat flow?
Heat will flow from the hotter object to the colder. The molecules in the hotter object will slow down and the molecules in the colder object will speed up. Eventually they will get to the point where they have the same temperature. This happens all the time around you.
Why does a thermometer work?
As the liquid gets hotter, it will expand and rise in the thermometer to show a higher temperature. It's the expansion and contraction due to temperature that allows the thermometer to work. When heat transfers from one object to another, this is called conduction. Some materials conduct heat better than others.
What state of matter is ice?
There are three states that matter can take depending on its temperature: solid, liquid, and gas. For example, if water is cold and its molecules are moving very slow, it will be a solid (ice). If it warms up some, the ice will melt and water becomes a liquid. If you add a lot of heat to water, the molecules will move very fast ...
What is the transfer of energy from one object to another due to a difference in temperature?
Heat is the transfer of energy from a one object to another due to a difference in temperature. Heat can be measured in joules, BTUs (British thermal unit), or calories.
When two things are combined or touching each other, their molecules will transfer energy called?
When two items are combined or touching each other, their molecules will transfer energy called heat. They will try to come to a point where they both have the same temperature. This is called equilibrium. Heat will flow from the hotter object to the colder. The molecules in the hotter object will slow down and the molecules in ...
Is metal a conductor of heat?
We use metal in pots and pans to cook because it will move the heat from the flame to our food quickly. Cloth, like a blanket, isn't a good conductor of heat.
How are temperature and heat different?
Heat is a type of energy that measures the total kinetic energy of the molecules in a body while the temperature is the state of being hot or cold of matter. Heat measurements are in Joules (J). On the other hand, temperature measurements are in degrees Celsius (°C).
What Is Heat?
Heat can be transferred through radiation, conduction, convection, and advection . Conduction is the transfer of heat between solids, radiation is the transfer of energy in the form of electromagnetic waves through air, or a medium and convention is a transfer of heat in liquids and gases. Advection, on the other hand, is the transfer of heat by flow of fluids from one point to another. Heat is in simple terms, transferred from a hot end to a less hot spot either independently or through a medium. The motion of particles of matter sparkled by heat increases when there is a transfer of heat. Latent heat is the amount of heat needed to change a solid into liquid or vapor, or change fluid into vapor without necessarily changing the temperature.
What is the difference between a thermometer and a colorimeter?
Heat is expressed as Q while expression for temperature is T. A thermometer is used to measure temperature while a colorimeter measures heat.
What is the purpose of a thermometer in an aquarium?
Thermometer in aquarium to measure the temperature of the water. Heat and temperature have since been confused to mean the same thing. It is, therefore, necessary to distinguish the two to bring out a clear picture of the two. They may sound similar but are much different according to science and research. Heat is a type of energy that measures the ...
What is the transfer of heat by flow of fluids from one point to another?
Heat is in simple terms, transferred from a hot end to a less hot spot either independently or through a medium. The motion of particles of matter sparkled by heat increases when there is a transfer of heat.
What is the temperature of a particle?
What Is Temperature? Absolute zero is the minimum temperature theoretically, at which particles remain motionless. Absolute zero is 0 K (Kelvin scale), -459.67 °F (Fahrenheit scale) and -273.15 °C (Celsius scale). Most parts of the world use the Celsius scale, but the United States usually uses the Fahrenheit scale.
What are the processes that change with temperature?
Temperature leads to change in phases or states of matter. Boiling, evaporation, melting, and freezing are all processes affected by changes in temperature. Heat and temperature are indeed different but casually mistaken to mean the same thing.