
What are phospholipids and why should you care?
Properties Of Phospholipids
- They are signal mediators.
- They are amphipathic molecules.
- They anchor proteins within the cell membranes.
- They are the major constituents of cell membranes.
- They are the components of bile and lipoproteins.
How do phospholipids benefit you?
Phospholipids. Phospholipids are essential in the body. In the most basic sense they are fat molecules. They are found in every cell in the human body and are a necessary building block. They form the cell membranes that allow nutrients to pass into the cells. EPA and DHA, the omega 3 fatty acids, are not absorbable by the human body in pure form.
What is the polar region of phospholipid?
The polar region (head) in the phosphate group of a phospholipid is attracted to water. The fatty acid tail is repelled by water. Phospholipids are a major and vital component of cell membranes.
Why do phospholipids form a bilayer in water?
When phospholipids are mixed with water, they spontaneously rearrange themselves to form the lowest free-energy configuration. This means that the hydrophobic regions find ways to remove themselves from water, while the hydrophilic regions interact with water. The resulting structure is called a lipid bilayer.
Why are phospholipid tails nonpolar?
In contrast, the interior of the membrane, between its two surfaces, is a hydrophobic or nonpolar region because of the fatty acid tails. This region has no attraction for water or other polar molecules (we will discuss this further in the next page).
Do phospholipids have a nonpolar tails?
Phospholipids are amphiphilic. They have a polar head and two hydrocarbon tails, which are nonpolar.
Are the tails of phospholipids hydrophobic?
Each phospholipid is amphipathic, with two hydrophobic tails and a hydrophilic head. The hydrophobic tails face inward towards one another, and the hydrophilic heads face outwards.
What are the nonpolar parts of phospholipids?
The hydrophobic, or “water-fearing,” part of a phospholipid consists of its long, nonpolar fatty acid tails. The fatty acid tails can easily interact with other nonpolar molecules, but they interact poorly with water.
Which part of a phospholipid is polar?
headsThe main component of the cell membrane is a phospholipid bi-layer or sandwich. The heads (the phospho part) are polar while the tails (the lipid part) are non-polar.
Why does the phospholipid has a polar head and non polar tail?
1: A phospholipid consists of a head and a tail. The "head" of the molecule contains the phosphate group and is hydrophilic, meaning that it will dissolve in water. The "tail" of the molecule is made up of two fatty acids, which are hydrophobic and do not dissolve in water.
Which characteristic describes the tails of phospholipids?
Which characteristic describes the tails of phospholipids? Correct Answer: Hydrophobic; The hydrocarbon tails of phospholipids tend to avoid contact with water, which helps drive the formation of the lipid bilayer.
What are the tails of phospholipids made of?
fatty acidsComposition of the Cell Membrane & Functions The phospholipids have a hydrophilic (water attracting) heads and two hydrophobic (water repelling) tails. The head of a phospholipid is made of an alcohol and glycerol group, while the tails are chains of fatty acids.
What makes the tail hydrophobic?
A hydrophobic tail is a hydrocarbon chain consisting of carbon and hydrogen atoms. It is an organic chain. It is composed of water-fearing fatty acid chains that restrict the movement of water molecules across the cell membrane. Molecules consisting of fatty acids are known to have hydrophobic tails.
What are the nonpolar parts of a phospholipid quizlet?
A phospholipid is made of a polar head (which includes the phosphate group and the glycerol molecules) and 2 nonpolar fatty acid tails. The head is hydrophilic and the tails are hydrophobic. Phospholipids make up cell membranes and form a bilayer.
Is hydrophilic polar or nonpolar?
If a molecule has areas where there is a partial positive or negative charge, it is called polar, or hydrophilic (Greek for "water-loving"). Polar molecules dissolve easily in water.
Which part is not water soluble in a phospholipid?
A phospholipid consists of a head and a tail. The “head” of the molecule contains the phosphate group and is hydrophilic, meaning that it will dissolve in water. The “tail” of the molecule is made up of two fatty acids, which are hydrophobic and do not dissolve in water.
Does a phospholipid have both polar and nonpolar regions?
The only molecule that contains both polar and non-polar regions from the ones given are phospholipids.
What are the tails of phospholipids made of?
fatty acidsComposition of the Cell Membrane & Functions The phospholipids have a hydrophilic (water attracting) heads and two hydrophobic (water repelling) tails. The head of a phospholipid is made of an alcohol and glycerol group, while the tails are chains of fatty acids.
What is the phospholipid tail made out of?
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule).
Is hydrophilic polar or nonpolar?
If a molecule has areas where there is a partial positive or negative charge, it is called polar, or hydrophilic (Greek for "water-loving"). Polar molecules dissolve easily in water.
Which side of the membrane are lipids arranged?
lipids are arranged within the membrane with polar head towards the outer side and non polar tails towards inner side, this ensures that the non polar tail is protected from aqueous environment.
Is water on the intracellular or extracellular side of the membrane?
There is water on the extracellular and intracellular side of the membrane. What's actually happening at a molecular dynamics level is the self-association of the hydrophobic lipid tail groups driven entropically by water. In other words the polar (hydro philic) head-groups "prefer" interacting with the water (called the interfacial region) and the the hydro phobic tail groups "prefer" not interacting with the water. With those two preferences in play, the lipid bilayer formation we know and love emerges.
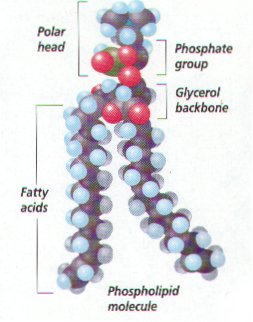
Phospholipid Definition
Phospholipid Structure
- A phospholipid consists of two basic parts: the head and the tail. The hydrophilic head consists of a glycerol molecule bound to a phosphate group. These groups are polar and are attracted to water. The second group, the hydrophobic tail, consists of two fatty acid chains. Some species use three fatty acid chains, but two is most common. The fatty acid chains can be saturated, or …
Phospholipid Function
- The amphiphilic nature of a phospholipid is extremely important for the functioning of cells. Every phospholipid, because of this dual relationship with water, is self-arranging when grouped together. The hydrophilic tails are drawn together via hydrophobic interactions, such as van der Waals forces. The hydrophilic heads are drawn toward the aqueous solution on either side of th…
Phospholipid Examples
- Cholesterol and Phospholipids
Within human cells, the balance of straight to bend phospholipid molecules, as well as sterol molecules, are important to the fluidity of cells. Cholesterolis especially important, helping to make cell membranes more rigid. Humans naturally produce cholesterol and do not need additional ch… - Drug Delivery using Phospholipid Micelles
The phospholipid molecules of the cell membrane are great at keeping substances out, but sometimes doctors want to get substances into a cell, to deliver a medicine or treatment. Many drugs now have phospholipid delivery systems. The drugs are either bound to the phospholipid …