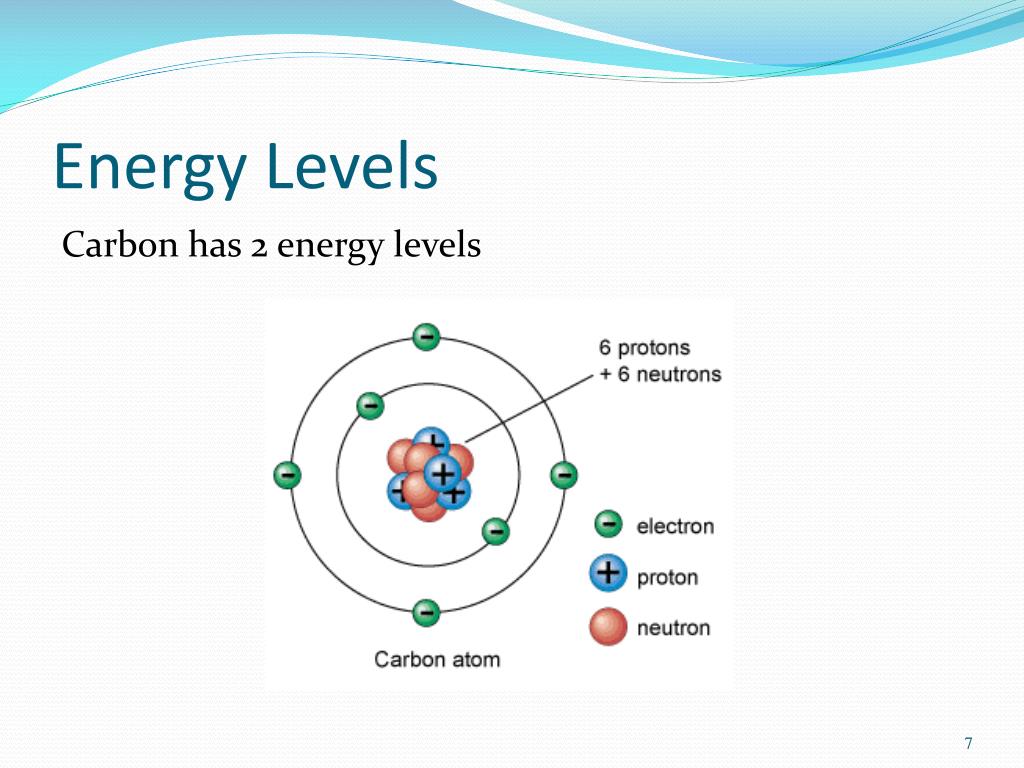
Summary
- The number of protons is always the same in atoms of the same element.
- The number of neutrons can be different, even in atoms of the same element.
- Atoms of the same element that contain the same number of protons, but different numbers of neutrons, are known as isotopes.
Are protons and neutrons always equal in number?
The number of protons in the nucleus of every atom of an element is always the same, but this is not the case with the number of neutrons. Atoms of the same element can have a different number of neutrons. Atoms want to have the same number of neutrons and protons but the number of neutrons can change. Click to see full answer
Does the number of protons equal neutrons?
Therefore, the number of neutrons is equal to 27 protons and neutrons minus 13 protons, which we can simplify to 27 minus 13 which gives us 14 neutrons. Which is the neutron number?
Are there more protons than neutrons in the universe?
Yes, there are about 7 times as many protons as neutrons. Since the universe is about 3/4 hydrogen by mass and 1/4 helium and hydrogen nuclei are primarily one proton and helium nuclei are primarily two protons and two neutrons, and protons and neutrons have about the same mass, you wind up with a 7:1 ratio of protons to neutrons.
What is the main difference between protons and neutrons?
What’s the main difference between protons and neutrons?, Protons are a type of subatomic particle with a positive charge. Protons are bound together in an atom’s nucleus as a result of the strong nuclear force. Neutrons are a type of subatomic particle with no charge (they are neutral).
See more
.PNG)
Are protons and neutrons the same or protons and electrons?
Protons: Positively charged subatomic particles located in the nucleus of an atom. Neutrons: Neutrally charged subatomic particles located in the nucleus of an atom. Electrons: Negatively charged subatomic particles located in orbitals surrounding the nucleus.
Why are protons and neutrons the same?
0:391:59Protons, Neutrons, and Electrons Explained | The Basics - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo neutrons are neutral as they have no charge they have a neutral charge. They also have a largeMoreSo neutrons are neutral as they have no charge they have a neutral charge. They also have a large mass in fact these two have a nearly equal mass the proton of the neutron. And they also are both in
Are protons neutrons and electrons always the same?
Even though electrons, protons, and neutrons are all types of subatomic particles, they are not all the same size. When you compare the masses of electrons, protons, and neutrons, what you find is that electrons have an extremely small mass, compared to either protons or neutrons.
Are protons and neutrons both electrons?
Key Concepts. Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Protons and neutrons are in the center of the atom, making up the nucleus. Electrons surround the nucleus.
Are protons and electrons equal?
The number of electrons on a neutral atom is equal to the number of protons in the nucleus of the atom. This is known as the atomic number, Z.
Which is heavier proton or neutron?
The neutron is very slightly heavier than the proton, by about 0.1%, or 1.00137841887 according to the best measurements.
Is neutron positive or negative?
Miller, a UW physics professor, has found that the neutron has a negative charge both in its inner core and its outer edge, with a positive charge sandwiched in between to make the particle electrically neutral.
How many neutrons are in an atom?
Since the vast majority of an atom's mass is found its protons and neutrons, subtracting the number of protons (i.e. the atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. In our example, this is: 14 (atomic mass) – 6 (number of protons) = 8 (number of neutrons).
Can an atom have no protons?
Neutronium is theoretically devoid of protons, so on face value it fits the bill, as no protons would mean no atomic number.
What is a proton made of?
Protons and neutrons are composed of two types: up quarks and down quarks. Each up quark has a charge of +2/3. Each down quark has a charge of -1/3. The sum of the charges of quarks that make up a nuclear particle determines its electrical charge.
How do u find neutrons?
To find the number of neutrons, subtract the number of protons from the mass number. number of neutrons=40−19=21.
Are protons positive or negative?
positive chargeA proton carries a positive charge (+) and an electron carries a negative charge (-), so the atoms of elements are neutral, all the positive charges canceling out all the negative charges.
How do protons and neutrons exist together?
Protons and neutrons exist together in an extremely small space with in the nucleus protons are positively charged particles where as neutrons are neutral particles having almost same mass as that of proton Neutrons and protons are bound in a nucleus by the short range strong nuclear force.
Why do protons and neutrons stay together in the nucleus?
Recall that protons are positively charged and repel each other by the electromagnetic force (a positive charge repels another positive charge). The reason that the positive nucleus doesn't fly apart is because of the strong nuclear force which acts between protons and neutrons and “glues” them together.
How are protons and neutrons similar and different?
In what ways are protons and neutrons alike? How are they different? They are different because protons have a positive charge and neutrons have a neutral charge. They are alike because they are both particles in a nucleus.
How are electrons and protons similar?
Electrons and protons are similar in that both are charged sub-atomic particles. There is an equal number of electrons and protons in the atoms of each element, which corresponds to the atomic number that has been assigned to the element.
What are the protons, neutrons, and electrons in an atom?
Key Takeaways: Number of Protons, Neutrons, and Electrons. Atoms are made of protons, neutrons, and electrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. A neutral atom has the same number of protons and electrons (charges cancel each other out).
Which atom has the same number of protons and electrons?
A neutral atom has the same number of protons and electrons (charges cancel each other out).
How many protons does a hydrogen atom have?
It's easy to get a hydrogen atom with one proton and one neutron (deuterium), yet you won't find a helium atom with an atomic weight of 2 because this would mean the helium atom had two protons and zero neutrons! If the atomic weight is 4.001, you can be confident the atom is helium, with 2 protons and 2 neutrons.
How to find the number of neutrons in an atom?
You can find the number of neutrons if you know the isotope of the atom. Simply subtract the number of protons (the atomic number) from the mass number to find the remaining neutrons.
How are elements defined?
Each element is defined by the number of protons found in each of its atoms. No matter how many electrons or neutrons an atom has, the element is defined by its number of protons. In fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).
What does it mean when an ion has a 2+ charge?
If an ion has a 2+ charge, like Zn 2+, this means there are two more protons than electrons. If the ion has a 1- charge (simply written with a minus superscript), then there are more electrons than the number of protons. For F -, the number of protons (from the periodic table) is 9 and the number of electrons is:
What element has 2 protons?
The element of an atom with 2 protons is always helium. If you are given the atomic weight of an atom, you need to subtract the number of neutrons to get the number of protons. Sometimes you can tell the elemental identity of a sample if all you have is the atomic weight.
Which particles have a positive charge equal to +1?
Protons are particles in the nucleus of an atom that have a positive charge equal to +1. Electrons are particles that have a negative charge equal to -1. Therefore, an element in a neutral state will have the same number of protons and electrons.
Why does an ion become positive when you remove electrons?
Because an electron has a negative charge, when you remove electrons, the ion becomes positive. When you add more electrons, the ion becomes negative.
What is the atomic number of an atom?
The atomic number is the proton. The number of electrons in an atom is always the same as the number of protons in the nucleus, unless it is an isotope. The neutron is the atomic mass minus the atomic number.
Why is an ion positive?
Although the number of protons in the atom remains the same, the number of electrons is altered in an ion. Because an electron has a negative charge, when you remove electrons, the ion becomes positive.
How to find the atomic number of an element?
Locate the element’s atomic number. The atomic number is located above the element symbol, in the upper left-hand corner of the square. The atomic number will tell you how many protons make up a single atom of an element. For example, boron (B) has an atomic number of 5, therefore it has 5 protons.
What are the three groups of elements?
Find your element on the periodic table. The table orders elements by atomic number and separates them into three main groups: metals, non-metals, and metalloids (semi-metals). Further elemental groupings include alkali metals, halogens, and noble gases.
Which type of charge is positively charged?
Protons are positively charged, electrons are negatively charged, and neutrons have no (neutral) charge.