It has a chirality center and therefore can exist as two enantiomers: The question is which one forms in excess, or in other words what is the stereochemistry of this, and in general, for the electrophilic addition reactions of alkenes In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. The words alkene and olefin are often used interchangeably. Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a …Alkene
What is the relationship between the enantiomers of alkenes?
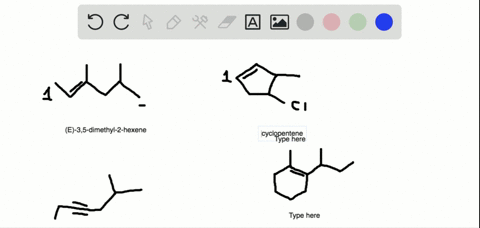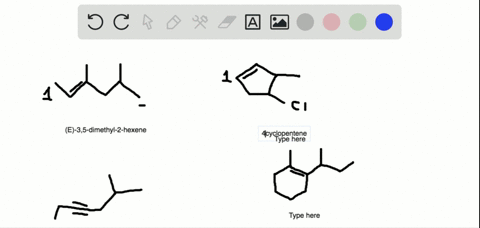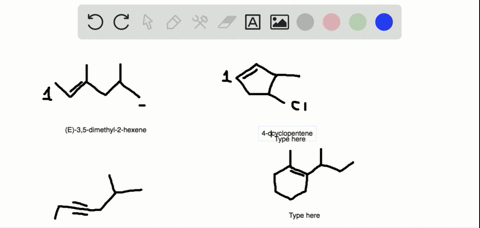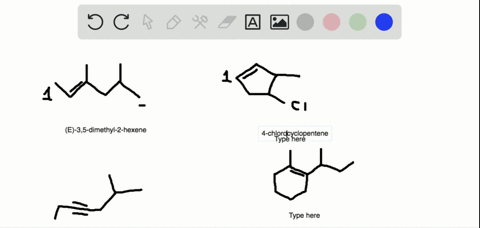
The first two molecules are a pair of enantiomers since they are nonsuperimposable mirror images. The second pair represents diastereomers since they are stereoisomers but not mirror images: So, what is the relationship between the two alkenes? They are clearly not identical because the atoms on the double bond are pointing at different directions.
Can alkenes be stereoisomeric?
Alkenes can be stereoisomeric even though this is slightly different than what we learn about enantiomers and diastereomers containing chirality centers. For example, let’s try to determine the relationship between molecules in each of the following pairs:
What is the difference between enantiomers and stereoisomers?
Enantiomers and diastereomers are the only two stereochemical relationships that you can have between any two molecules. The stereoisomers are any two molecules that fulfill the following two requirements: Both molecules must have the same atom connectivity. So, what’s the difference then?
What are enantiomers and diastereomers in organic chemistry?
Organic Chemistry Enantiomers and Diastereomers Enantiomers and diastereomers are the only two stereochemical relationships that you can have between any two molecules. The stereoisomers are any two molecules that fulfill the following two requirements: Both molecules must have the same molecular formula, and

Why are cis and trans designations included in the nomenclature of alkenes?
The cis and trans designation is included in the nomenclature of alkenes to distinguish the stereochemistry.
Which isomer has substituents on opposite sides of the ring?
The substituents of the cis isomer are on the same side of the ring while the trans isomer has them on opposite sides of the ring:
What happens if two hydrogens are on the same side?
And this is similar to what we discussed about double bonds with different alkyl groups. Remember, in this case we focus on the orientation of hydrogens. If both hydrogens are on the same side, it is cis and if they are on opposite sides, then we have a trans isomer.
Why is the origin of the cis and trans isomerism locked?
It locked because there is no rotation around the double bond and this, in turn, means that we cannot switch the orientation of the groups on the double bond.
What are the first two molecules?
The first two molecules are a pair of enantiomers since they are nonsuperimposable mirror images. The second pair represents diastereomers since they are stereoisomers but not mirror images:
Is a cis isomer stereoisomer?
Therefore, cis and trans isomers are stereoisomers. This comparison brings an interesting question of whether we can ever have cis – trans isomerism for a system with single bonds. And the answer is yes, there are cis and trans isomers for systems with sigma bonds. The restricted rotation about the single bonds in cyclic systems makes it possible ...
Can an alkene be stereoisomeric?
If any of the carbons in the double bond is connected to two identical groups, the alkene cannot be cis or trans. That is to say, the alkene cannot be stereoisomeric. For example, the following alkene has two ethyl groups on the right carbon, and we cannot say that there are two ethyl groups on the same or on the opposite sides of the double bond:
