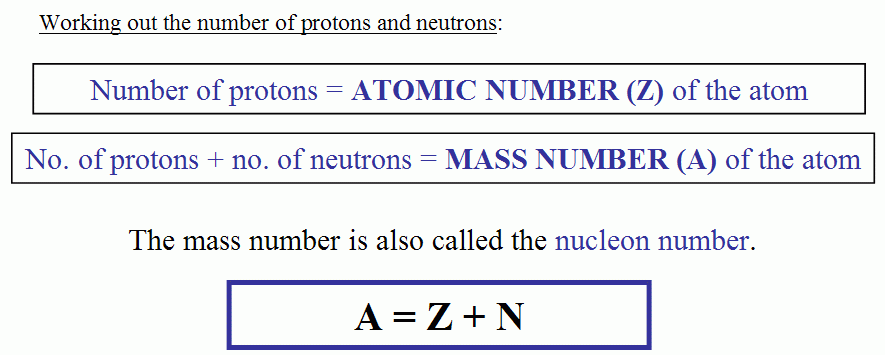How do you find the number of neutrons on the periodic table?
Jan 20, 2020 · Can you tell from the periodic table how many neutrons are in an atom? The number of neutrons can be found simply by identifying the atomic mass (found at the bottom of the element on the periodic table) and the atomic number. Subtract the atomic number from the atomic mass. Click to see full answer.
Why do we know the number of neutrons in an atom?
If you want to know the average number of neutrons for any element, just take the molar mass, and subtract the number of protons from that number. For example Gold has a mass of 196.97, and it has 79 protons, so 196.97–79=117.97. So Gold has on average 118 neutrons.
How do you determine the number of electrons in a neutral atom?
Jun 02, 2019 · The periodic table lists the atomic weight for each element, which can be used to find mass number, For hydrogen, for example, the atomic weight is 1.008. Each atom has an integer number of neutrons, but the periodic table gives a decimal value because it is a weighted average of the number of neutrons in the isotopes of each element. So, what you need to do is …
Does the number of neutrons in an ionic compound matter?
Remember that the numbers above the symbol are the mass numbers, so Cl-35 has a total of 35 protons and neutrons, and Cl-37 has a total of 37 protons and neutrons. Since we know the atomic number of Cl is 17, we can work out that Cl-35 has 18 neutrons, and Cl …

Can you tell from the periodic table exactly how many neutrons are in an atom quizlet?
How do u know how many neutrons an element has?
Since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e. the atomic number) from the atomic mass will give you the calculated number of neutrons in the atom.
Which is the neutron number?
How do you find the protons neutrons and electrons of an element?
- number of protons = atomic number.
- number of electrons = atomic number.
- number of neutrons = mass number - atomic number.
How to find the number of neutrons in an atom?
Categories: Chemistry. Article Summary X. To find the number of neutrons in an atom, start by locating the element on the periodic table. Then, find the element's atom number, which is usually in the top left or middle of the box above the element symbol.
What is the atomic number of an element?
The atomic number is the number of protons in a single atom of that element. Os is number 76, meaning one atom of osmium has 76 protons. The proton number never changes in an element; it's basically what makes that element that element.
What is the empty space between the atomic cloud of an atom and its nucleus?
The empty space between the atomic cloud of an atom and its nucleus is just that: empty space, or vacuum. That's the simple answer, but there are a few subtleties: Subatomic particles such as electrons, protons and neutrons need to be treated as quantum objects.
What does the number after the decimal point mean?
The numbers after the decimal point represent the usually very small mass of the electrons in the atom. In our example, this is: 190 (atomic weight) – 76 (number of protons) = 114 (number of neutrons).
How to find the number of neutrons in an atom?
You can find the number of neutrons if you know the isotope of the atom. Simply subtract the number of protons (the atomic number) from the mass number to find the remaining neutrons.
What are the protons, neutrons, and electrons in an atom?
Key Takeaways: Number of Protons, Neutrons, and Electrons. Atoms are made of protons, neutrons, and electrons. Protons carry a positive electrical change, while electrons are negatively charged, and neutrons are neutral. A neutral atom has the same number of protons and electrons (charges cancel each other out).
What are the three parts of an atom?
The three parts of an atom are positive-charged protons, negative-charged electrons, and neutral neutrons. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element.
How many protons does a hydrogen atom have?
It's easy to get a hydrogen atom with one proton and one neutron (deuterium), yet you won't find a helium atom with an atomic weight of 2 because this would mean the helium atom had two protons and zero neutrons! If the atomic weight is 4.001, you can be confident the atom is helium, with 2 protons and 2 neutrons.
Which atom has more protons than electrons?
A cation carries a positive charge and has more protons than electrons. An anion carries a negative charge and has more electrons than protons. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. The number of protons of an atom cannot change via any chemical reaction, ...
Which atom has a negative charge?
An anion carries a negative charge and has more electrons than protons. Neutrons do not have a net electric charge, so the number of neutrons does not matter in the calculation. The number of protons of an atom cannot change via any chemical reaction, so you add or subtract electrons to get the correct charge.
How many protons does zinc have?
For zinc, the number of protons is 30 . The element of an atom with 2 protons is always helium. If you are given the atomic weight of an atom, you need to subtract the number of neutrons to get the number of protons. Sometimes you can tell the elemental identity of a sample if all you have is the atomic weight.
How to find neutrons in a periodic table?
To find the neutrons in an element of the periodic table, you need two things, the atomic number of the element and the atomic mass of that element. See below
What does the atomic number represent?
The atomic number represents the amount of protons in an element and is on top of the symbol for that element...this is always a whole number. Right below the chemical symbol, you will find a number that usually has decimals and is not a whole number...this is the atomic mass of the element and it represents the number of protons ...
How to find neutrons in an element?
To find the number of neutrons, subtract the element’s atomic number from its atomic mass (the number listed underneath the element).
How to find the number of neutrons in a molecule?
To find the number of neutrons, you will need to subtract the atomic number from the atomic mass. Remember that the atomic number is the same as the number of protons, which you have already identified. For our boron example, 11 (atomic mass) – 5 (atomic number) = 6 neutrons.
What is the periodic table?
The periodic table is a chart that organizes elements by their atomic structure. It is color-coded and assigns each element a unique 1 or 2-letter abbreviation. Other elemental information includes atomic weight and atomic number. You can find a periodic table online or in a chemistry book.
What are the three groups of elements in the periodic table?
The table orders elements by atomic number and separates them into three main groups: metals, non-metals, and metalloids (semi-metals). Further elemental groupings include alkali metals, halogens, and noble gases. Using the group (columns) or period (rows) can make the element easier to locate on the table.
How to find the atomic number of an element?
Locate the element’s atomic number. The atomic number is located above the element symbol, in the upper left-hand corner of the square. The atomic number will tell you how many protons make up a single atom of an element. For example, boron (B) has an atomic number of 5, therefore it has 5 protons.
Where is the atomic number located?
The atomic number is located above the element symbol, in the upper left-hand corner of the square. The atomic number will tell you how many protons make up a single atom of an element. For example, boron (B) has an atomic number of 5, therefore it has 5 protons.
Which particles have a positive charge equal to +1?
Protons are particles in the nucleus of an atom that have a positive charge equal to +1. Electrons are particles that have a negative charge equal to -1. Therefore, an element in a neutral state will have the same number of protons and electrons.