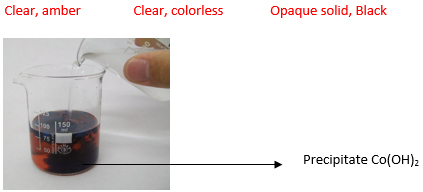
Not necessarily. There are a variety of spontaneous double displacement reactions. Some but not all of them involve the production of a precipitate. For example, the neutralization reaction between hydrochloric acid HCl and sodium hydroxide NaOH counts as a double displacement reaction. However, this reaction produces no precipitate.
What is a double displacement reaction?
Updated May 09, 2018. A double displacement reaction is a type of reaction where two reactants exchange ions to form two new compounds. Double displacement reactions typically result in the formation of a product that is a precipitate.
What are some examples of double replacement reactions?
Definition and examples of double replacement reactions. Predicting and balancing neutralization and precipitation reactions. What is a double replacement reaction? Double replacement reactions —also called double displacement, exchange, or metathesis reactions —occur when parts of two ionic compounds are exchanged, making two new compounds.
What type of reaction is precipitation reaction?
Precipitation reactions belong to a general class of reactions called double-displacement reactions. Double displacement reactions have the following form. Note that the elements in two reacting compounds change partners. AB + CD → AD + CB
How do you identify a neutralization reaction as double displacement?
The initial neutralization reaction is: You'll notice the cations exchanged anions, but the way the compounds are written, it's a bit trickier to notice the anion swap. The key to identifying the reaction as double displacement is to look at the atoms of the anions and compare them on both sides of the reaction.

Are all double displacement reactions precipitate reaction?
All double displacement reactions are not precipitation reactions.
Do Double replacement reactions form precipitates?
A double-replacement reaction exchanges the cations (or the anions) of two ionic compounds. A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate.
Why do precipitates form in some double displacement reactions?
Formation of a Precipitate A precipitate forms in a double-replacement reaction when the cations from one of the reactants combine with the anions from the other reactant to form an insoluble ionic compound.
Which of the following is an example of double displacement reaction but not precipitation reaction?
AgNO3 + HCl ⇌ AgCl + HNO.
Can a displacement reaction precipitate form?
Double displacement reactions typically result in the formation of a product that is a precipitate.
What is not formed during a double replacement reaction?
Explanation: A double displacement (double replacement) reaction occurs when two aqueous ionic solutions react to produce a solid precipitate, an insoluble gas, or water. If none of these are produced, then a double displacement did not take place.
What is the difference between double decomposition and precipitation reaction?
1)Those reactions in which 2 compounds react by an exchange of ions to form 2 new compound, called double displacement reaction. 1) any reaction in which an insoluble solid is formed that separates from solution is called a precipitation reaction. White precipitation is formed in this reaction.
How do you know a double displacement reaction has occurred?
0:0010:49Chemical Reactions (1 of 11) Double Replacement Reactions ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipOkay in today's video I'm going to go over double displacement or double replacement reactionsMoreOkay in today's video I'm going to go over double displacement or double replacement reactions double replacement reactions are when there is an exchange of positive ions between two compounds. And so
Which product in the reaction forms a precipitate?
The definition of a precipitation reaction is when two (or more) soluble salts react to form an insoluble product. The reactants are ions in the solution. The insoluble product is referred to as precipitate.
Why the double displacement of HCl and NaOH is not a precipitation reaction?
When both the products of a double displacement reaction are water soluble, then no precipitate will occur and there will not be a visible product. Acid-base reactions like that between HCl and NaOH in solution are double displacement reactions in which both the products formed are soluble in water.
What are double displacement reaction and precipitation reaction give one example of each reaction?
Double displacement reactions generally take place in aqueous solutions in which the ions precipitate and there is an exchange of ions. For example, on mixing a solution of barium chloride with sodium sulphate, a white precipitate of barium sulphate is immediately formed. These reactions are ionic in nature.
What are the types of double displacement reaction?
There are two types of double displacement reactions: precipitation and neutralization.
What happens in a double-replacement reaction?
A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. Double replacement reactions are also called double replacement reactions, double displacement reactions, or metathesis reactions.
What kind of reaction is precipitation?
A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement, double replacement, or metathesis reactions.
Which product in the reaction forms a precipitate?
The definition of a precipitation reaction is when two (or more) soluble salts react to form an insoluble product. The reactants are ions in the solution. The insoluble product is referred to as precipitate.
Can two aqueous solutions make two precipitates?
Yes is the answer to your question provided that reactant two molecules are salts. Two salts can react with eact other and form two new stable salts depending on their Ksp values.
What is double displacement reaction?
A double displacement reaction is a type of reaction in which two reactants exchange ions to form two new compounds. Double displacement reactions typically result in the formation of a product that is a precipitate .
What type of bond is formed in a double displacement reaction?
The reaction occurs most often between ionic compounds, although technically the bonds formed between the chemical species may be either ionic or covalent in nature. Acids or bases also participate in double displacement reactions. The bonds formed in the product compounds are the same type of bonds as seen in the reactant molecules.
What is the reaction that produces a salt?
Neutralization reactions are double displacement reactions between acids and bases. When the solvent is water, a neutralization reaction typically produces an ionic compound —a salt.
What are the two types of reactions in chemistry?
The two types most commonly encountered in chemistry classes are precipitation reactions and neutralization reactions. A precipitation reaction occurs between two aqueous ionic compounds to form a new insoluble ionic compound. Here's an example reaction between lead (II) nitrate and potassium iodide to form (soluble) potassium nitrate and ...
What is the reaction between vinegar and baking soda?
The reaction between vinegar and baking soda in the classic baking soda volcano is an example of a neutralization reaction. This particular reaction then proceeds to release a gas ( carbon dioxide ), which is responsible for the fizz that results.
What is the reaction between silver and sodium?
The reaction between silver nitrate and sodium chloride is a double displacement reaction. The silver trades its nitrite ion for the sodium's chloride ion, causing the sodium to pick up the nitrate anion.#N#AgNO 3 + NaCl → AgCl + NaNO 3
What is a double replacement reaction?
Double replacement reactions —also called double displacement, exchange, or metathesis reactions —occur when parts of two ionic compounds are exchanged, making two new compounds. The overall pattern of a double replacement reaction looks like this:
What is precipitation reaction?
A precipitation reaction is when two aqueous ionic compounds form a new ionic compound that is not soluble in water. One example is the reaction between lead (II) nitrate and potassium iodide. Both compounds are white solids that can be dissolved in water to make clear, colorless solutions. When you combine the two clear solutions, you get the following reaction:
What is the insoluble product compound called?
The insoluble product compound is called the precipitate . The solvent and soluble components of the reaction are called the supernatant or supernate. We can use solubility rules to predict whether a precipitation reaction will take place. The formation of a solid precipitate is the driving force that makes the reaction proceed in the forward direction.
Can double replacement reactions be written as molecular, complete ionic, or net ionic equations?
Note that double replacement reactions can be written as molecular, complete ionic, or net ionic equations. In this article we are only writing out the molecular equation, but you probably want to be familiar with writing the other forms of the equation as well.
Can you swap cations and anions?
You can think of the reaction as swapping the cations or the anions, but not swapping both since you would end up with the same substances you started with. The solvent for a double replacement reaction is usually water, and the reactants and products are usually ionic compounds—but they can also be acids or bases.

Alternative Terms
Double Displacement Reaction Examples
- The reaction between silver nitrate and sodium chloride is a double displacement reaction. The silver trades its nitrite ion for the sodium's chloride ion, causing the sodium to pick up the nitrate anion. AgNO3 + NaCl → AgCl + NaNO3 Here's another example: BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2 NaCl(aq)
How to Recognize A Double Displacement Reaction
- The easiest way to identify a double displacement reaction is to check to see whether or not the cations exchanged anions with each other. Another clue, if the states of matter are cited, is to look for aqueous reactants and the formation of one solid product (since the reaction typically generates a precipitate).
Types of Double Displacement Reactions
- Double displacement reactions may be classified into several categories, including counter-ion exchange, alkylation, neutralization, acid-carbonate reactions, aqueous metathesis with precipitation (precipitation reactions), and aqueous metathesis with double decomposition (double decomposition reactions). The two types most commonly encountered in chemistry cla…
Sources
- Dilworth, J. R.; Hussain, W.; Hutson, A. J.; Jones, C. J.; Mcquillan, F. S. (1997). "Tetrahalo Oxorhenate Anions."Inorganic Syntheses, vol. 31, pp. 257–262. doi:10.1002/9780470132623.ch42
- IUPAC. Compendium of Chemical Terminology(2nd ed.) (the "Gold Book"). (1997).
- March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(3r…
- Dilworth, J. R.; Hussain, W.; Hutson, A. J.; Jones, C. J.; Mcquillan, F. S. (1997). "Tetrahalo Oxorhenate Anions."Inorganic Syntheses, vol. 31, pp. 257–262. doi:10.1002/9780470132623.ch42
- IUPAC. Compendium of Chemical Terminology(2nd ed.) (the "Gold Book"). (1997).
- March, Jerry (1985). Advanced Organic Chemistry: Reactions, Mechanisms, and Structure(3rd ed.). New York: Wiley. ISBN 0-471-85472-7.
- Myers, Richard (2009). The Basics of Chemistry. Greenwood Publishing Group. ISBN 978-0-313-31664-7.