A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products. Usually, a double displacement reaction results in precipitate formation. The chemical bonds between the reactants may be either covalent or ionic.
What is the difference between precipitation and double replacement reactions?
A double-replacement reaction exchanges the cations (or the anions) of two ionic compounds. A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate. Solubility rules are used to predict whether some double-replacement reactions will occur.
What are some examples of double replacement reactions?
Definition and examples of double replacement reactions. Predicting and balancing neutralization and precipitation reactions. What is a double replacement reaction? Double replacement reactions —also called double displacement, exchange, or metathesis reactions —occur when parts of two ionic compounds are exchanged, making two new compounds.
What type of reaction is a precipitate?
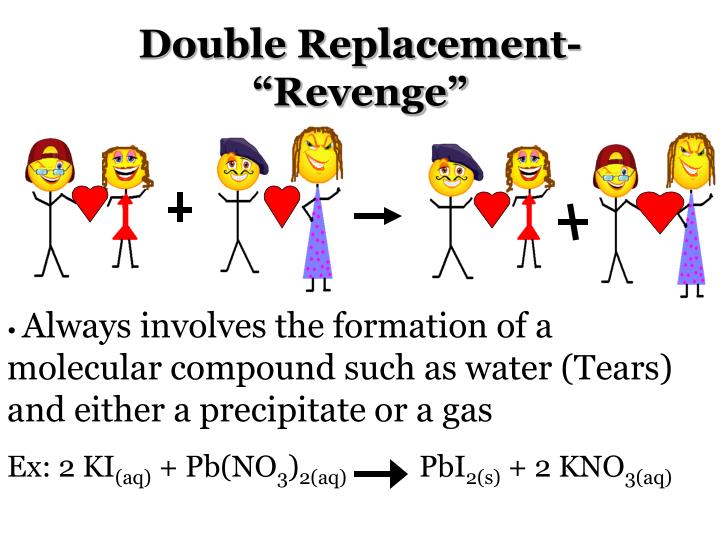
A precipitate forms in a double-replacement reaction when the cations from one of the reactants combine with the anions from the other reactant to form an insoluble ionic compound. When aqueous solutions of potassium iodide and lead (II) nitrate are mixed, the following reaction occurs.
What happens when a double displacement reaction occurs?
When a double displacement reaction occurs, the cations and anions switch partners, resulting in the formation of two new ionic compounds AD and CB, one of which is in the solid state. This solid product is an insoluble ionic compound called a precipitate. Click to see full answer. Then, are all precipitation reactions double displacement?

What is the reaction that forms a precipitate?
A precipitate forms in a double-replacement reaction when the cations from one of the reactants combine with the anions from the other reactant to form an insoluble ionic compound. When aqueous solutions of potassium iodide and lead (II) nitrate are mixed, the following reaction occurs.
What are some examples of double displacement reactions?
For example, on mixing a solution of barium chloride with sodium sulphate, a white precipitate of barium sulphate is immediately formed.
What is double displacement?
A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products. Usually, a double displacement reaction results in precipitate formation. The chemical bonds between the reactants may be either covalent or ionic.
What are the solubility rules used to predict?
Solubility rules are used to predict whether some double-replacement reactions will occur. Also Know, what does a double replacement reaction produce? A double replacement reaction is a chemical reaction where two reactant ionic compounds exchange ions to form two new product compounds with the same ions. Additionally, why does a precipitate form ...
Is a precipitation reaction always double displacement?
Consequently, are precipitation reactions always double displacement? A double-replacement reaction exchanges the cations (or the anions) of two ionic compounds. A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate. Solubility rules are used to predict whether some double-replacement reactions will ...
