
It's often said that every element was made in a star, but there's more to it than that.
- Big Bang fusion. Just a few seconds after the Big Bang, everything was too hot to be anything. ...
- Exploding massive stars. ...
- Dying low-mass stars. ...
- Cosmic ray fission. ...
- Merging neutron stars. ...
- Exploding white dwarf stars. ...
- Human synthesis. ...
Was every element made in a star?
It's often said that every element was made in a star, but there's more to it than that. Where does the aluminum foil in your kitchen come from? It's mined from the earth, of course, but before how did it get there? All of the elements in the universe have very disparate sources and were produced under very different conditions.
Where did the elements in your body come from?
Where Your Elements Came From The hydrogen in your body, present in every molecule of water, came from the Big Bang. There are no other appreciable sources of hydrogen in the universe. The carbon in your body was made by nuclear fusion in the interior of stars, as was the oxygen.
Where did the elements come from the Big Bang?
The Big Bang, for instance, made hydrogen, helium, and lithium; where did the other elements come from? Scientists know enough to say with some certainty what percentage of a given element came from, say, colliding neutron stars, supernovae from massive stars, or cosmic rays.
How do stars make heavy elements?
Brick by brick, element by element, nuclear processes in stars take the abundant hydrogen atoms and build heavier elements, from helium and carbon all the way to technetium and beyond.
Are all elements created by stars?
Virtually all of the elements we see on the Periodic Table were made at some point during the life and death of a star. Only hydrogen, helium, and lithium were created in a different way, i.e., they were created as a result of the Big Bang explosion.
Where do all elements come from?
the universe formed through the big bang explosion, all of the elements on Earth have been cooked for billions of years in stars and then released in the universe through super- nova explosions.
What elements are not created in stars?
In fact, you can't make the first of the heavier-than-helium elements in stars at all. And yet, lithium, beryllium and boron not only exist, but boron in particular is vital for life-as-we-know-it on Earth. Without boron, there would be no such thing as a cell wall, and hence, no such thing as a plant.
Why are all elements not produced by All stars?
Low-mass stars don't have enough energy to directly produce heavier elements up to iron like massive stars do, and they don't explode in supernovae to produce elements heavier than iron.
Does iron come from stars?
Iron is made inside stars, specifically red super-giants. The elements form together inside a star during fusion. When the supernova occurs, the iron fragments are blasted into the space. This is how Iron came to Earth millions of years ago.
What are humans made of?
Almost 99% of the mass of the human body is made up of six elements: oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus. Only about 0.85% is composed of another five elements: potassium, sulfur, sodium, chlorine, and magnesium. All 11 are necessary for life.
Where did the first elements come from?
The early universe (left) was too hot for electrons to remain bound to atoms. The first elements — hydrogen and helium — couldn't form until the universe had cooled enough to allow their nuclei to capture electrons (right), about 380,000 years after the Big Bang.
Is it possible to create a new element?
You can not create new elements by mixing different compounds. In order to create a new element you have to change the number of protons in a nucleus. It is possible to do this but it requires bombarding various elements, one with the other, by means of high energy particle accelerators.
Where did heavy elements come from?
Heavy elements are produced during stellar explosion or on the surfaces of neutron stars through the capture of hydrogen nuclei (protons). This occurs at extremely high temperatures, but at relatively low energies.
Why can stars only make iron?
If the reaction rates are high, then a net flux of energy is produced. Fusion of elements with mass numbers (the number of protons and neutrons) greater than 26 uses up more energy than is produced by the reaction. Thus, elements heavier than iron cannot be fuel sources in stars.
Where did the first elements come from?
The early universe (left) was too hot for electrons to remain bound to atoms. The first elements — hydrogen and helium — couldn't form until the universe had cooled enough to allow their nuclei to capture electrons (right), about 380,000 years after the Big Bang.
How do elements are made?
An element is any substance made up entirely of one particular type of atom – the basic building blocks of stuff. We know that elements have three ingredients: protons, neutrons, and electrons. These are some of the tiniest particles in nature.
Does everything come from hydrogen?
Approximately 73% of the mass of the visible universe is in the form of hydrogen. Helium makes up about 25% of the mass, and everything else represents only 2%.
Where can you find all the elements?
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom.
Where do elements come from?
In the early 1950s, it was still unclear how the elements that make up our universe, our solar system, even our human bodies, were created. Initially, the most popular scenario was that they were all made in the Big Bang.
What element was discovered in the sky 70 years ago?
Nearly 70 years ago, astronomer Paul Merrill was watching the sky through a telescope at Mount Wilson Observatory in Pasadena, California. As he observed the light coming from a distant star, he saw signatures of the element technetium.
How is technetium created?
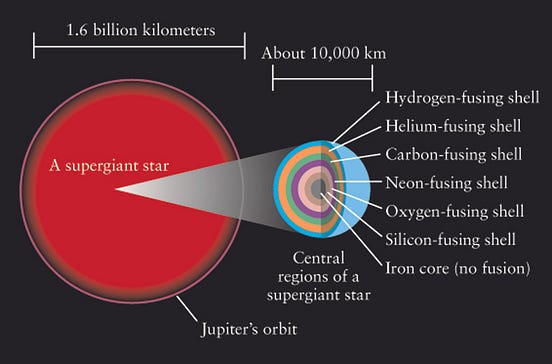
Technetium is created in the shell. ESO, CC BY-ND. These nuclear reactions serve two purposes. First, they release energy that heats the star , providing the outward pressure that prevents its gravitational collapse and keeps the star in balance for billions of years. Second, they fuse light elements into heavier ones.
What did Merrill discover about the universe?
On May 2, 1952, Merrill reported his discovery in the journal Science. Among the three interpretations offered by Merrill was the answer: Stars create heavy elements! Not only had Merrill explained a puzzling observation, he had also opened the door to understand our cosmic origins.
Why are nuclear reactions so difficult to study?
And they’re extremely difficult to study in the laboratory because nuclei are hard to fuse. That’s why, for more than six decades, nuclear physicists have continued to work to get a handle on the nuclear reactions that drive the stars.
Why does a star compress?
As the star compresses because of its gravity, though, the temperature at its center increases. In such hot conditions, now when nuclei run into each other they have enough energy to merge together. This is what physicists call a nuclear fusion reaction. Fusion reactions happen in different parts of a star.
What is the name of the study of how the elements are synthesized?
Merrill’s discovery marked the birth of a completely new field: stellar nucleosynthesis. It’s the study of how the elements, or more accurately their atomic nuclei, are synthesized in stars.
How did hydrogen and helium form?
Hydrogen formed pretty much instantly and even helium (with nuclei containing 2 protons) formed in relatively short order (part of a process referred to as Big Bang nucleosynthesis). As this hydrogen and helium began to form in the early universe, there were some areas where it was denser than in others.
What does helium and titanium produce?
Titanium plus helium produces chromium . Chromium plus helium produces iron. Other fusion pathways create the elements with odd numbers of protons. Iron has such a tightly bound nucleus that there isn't further fusion once that point is reached. Without the heat of fusion, the star collapses and explodes in a shockwave.
What is the simplest atom in the universe?
The simplest type of atom in the universe is a hydrogen atom, which contains a single proton in the nucleus (possibly with some neutrons hanging out, as well) with electrons circling that nucleus. These protons are now believed to have formed when the incredibly high energy quark-gluon plasma of the very early universe lost enough energy that quarks began bonding together to form protons (and other hadrons, like neutrons). Hydrogen formed pretty much instantly and even helium (with nuclei containing 2 protons) formed in relatively short order (part of a process referred to as Big Bang nucleosynthesis).
How is helium fusion made?
Largely, it is fused into carbon via the triple-alpha process in which three helium-4 nuclei (alpha particles) are transformed. The alpha process then combines helium with carbon to produce heavier elements, but only those with an even number of protons.
How long does it take for a star to burn?
The energy released during this process is what causes the sun (or any other star, for that matter) to burn. It takes nearly 10 million years to burn through the hydrogen and then things heat up and the helium begins fusing.
What is the process of atoms forming in space?
Once these clouds became large enough, they were drawn together by gravity with enough force to actually cause the atomic nuclei to fuse, in a process called nuclear fusion.
What happens when a star is fusioned?
Fusion inside stars transforms hydrogen into helium, heat, and radiation. Heavier elements are created in different types of stars as they die or explode.
Where does the process of creating elements occur?
The process of creating the elements occurs in the heart of the star as it heads towards its last days - in a process known as stellar nucleosynthesis. This method is responsible for how many of the heavier elements that we see around us (including oxygen, carbon, calcium, nitrogen, phosphorus and more) are created, and is known as the slow-neutron capture process, or s-process.
Which element is a type Ia supernova?
In this latest paper, Dr Panther and her team describe how the nucleosynthesis of the element Chromium occurs in this niche group of Type Ia supernovae, which rapidly decays into the element Vanadium, releasing gamma-rays in the process and affecting the intensity of the light of the supernova. The team studied a simulation of supernovae that produced such gamma-rays to determine how they can be best observed with telescopes, tuned into detecting high-energy frequencies from astrophysical sources.
Why are supernovae used as cosmic candles?
Because the standard Chandrasekhar limit dictates how big this type of supernova will be (thus producing a roughly standardised measure for each event), then these supernovae can be used as “cosmic candles” to measure distances across the universe by figuring out how far away the supernovae are.
What happens when supernovae occur?
When supernovae occur, and material is being rapidly expelled from the star, atoms and nuclei can collide to form different elements. This is how some of the bigger elements in our universe have been created, with this the method is known as the rapid neutron capture process, or r-process. Heavy elements are also created via the r-process in the event of neutron star merger events, known as kilonovae.
What can light curves tell us about supernovae?
Observations of these light curves can tell us lots about how supernovae events happen , about the localised environment surrounding the explosion, and what elements are being created in the process , thanks to people like Dr Pankey.
What is a supernova?
Supernovae are explosive events that result in the materials and gases of a star being flung far and wide into the interstellar medium, later going on to become future generations of new stars, planets, moons, and even living beings.
Is a white dwarf a stable star?
By itself, a white dwarf is a fairly stable star, but as it steals material from its companion it becomes larger and more massive.
Where did the elements in our body come from?
Where Your Elements Came From. The hydrogen in your body, present in every molecule of water, came from the Big Bang. There are no other appreciable sources of hydrogen in the universe. The carbon in your body was made by nuclear fusion in the interior of stars, as was the oxygen. Much of the iron in your body was made during supernovas ...
Why is the periodic table color coded?
The featured periodic table is color coded to indicate humanity's best guess as to the nuclear origin of all known elements. The sites of nuclear creation of some elements, such as copper, are not really well known and are continuing topics of observational and computational research.
What is gold made of?
The gold in your jewelry was likely made from neutron stars during collisions that may have been visible as short-duration gamma-ray bursts or gravitational wave events. Elements like phosphorus and copper are present in our bodies in only small amounts but are essential to the functioning of all known life.
What are the elements that make up cosmic rays?
Next, we have 3 elements under the category of “Cosmic Rays:” lithium, berylium, and boron.
What are the elements that are classified under the Big Bang?
Hydrogen and helium are the only elements that (as you can see) are classified under “ Big Bang .”. There are no other appreciable sources of this in the universe, thus, they are alone in this category. Let’s take a clear look at the image before moving on with the explanation:
Is the periodic table the first table of elements?
Interestingly, the periodic table, as we know it today, isn’t the first table of elements . In fact, we once believed that all things in the universe were made from just Earth, Fire, Water, and Air (thankfully, we didn’t stick with that organizational structure).
Is the periodic table solid?
Of course, today, the Table is a lot more solid and well established than it was when it was first developed. But that is because, at that time, many elements weren’t discovered yet. Conversely, these days, we already have an abundance of elements—meaning that most all naturally occurring elements on planet Earth have already been discovered and the Periodic Table has been mostly filled in. Mostly.
Why are the elements in the universe ionic?
The elements are in ionic form because the universe is still very hot— too hot to form atoms.
How does a star spend 90% of its lifetime fusing hydrogen into helium?
A star spends 90% of its lifetime fusing hydrogen into helium. After the hydrogen has been depleted, it begins fusing helium into higher elements. With each new stage of elemental fusion, the core becomes denser, and the outer layers of the stars begin to expand, gradually turning into a red giant.
How does a star burn fuel?
A star spends 90% of its lifetime fusing hydrogen into helium.
How did stars keep their cores from collapsing?
To keep their cores from collapsing under gravity, the stars needed to tap into a constant source of energy. This energy was eagerly provided by the release of binding energy.
Why are elements in ionic form?
The elements are in ionic form because the universe is still very hot—too hot to form atoms. Approximately 380,000 years after the Big Bang was the epoch of recombination. After years of expanding and cooling the universe was finally ready for the nuclei to capture the electrons.
What was the temperature of the universe after the Big Bang?
Immediately after the Big Bang, the universe was a dense soup of matter and energy. The temperature was around 10 32 Kelvin. The universe started inflating and simultaneously cooling off (though it was still trillions of Kelvin). The elementary particles (quarks and electrons) started popping into existence.
Why is the periodic table important?
The periodic table makes our lives much easier, but also more difficult, all at the same time! It not only helps us remember and understand our elements, but also gives rise to deep existential questions, such as how did these elements come into existence at all?
Why are we made of star stuff?
Because humans and every other animal as well as most of the matter on Earth contain these elements, we are literally made of star stuff, said Chris Impey, professor of astronomy at the University of Arizona.
What is the name of the song that says we are all made of stars?
The remains of a once-explosive supernova illuminate part of a nearby galaxy in this image taken by the Hubble Space Telescope. (Image credit: NASA/ESA/HEIC/Hubble Heritage Team) The theory that everyone and everything on Earth contains minuscule star particles dates back further than Moby's popular 2002 song "We Are All Made of Stars.".
What happens when a star explodes?
When it has exhausted its supply of hydrogen, it can die in a violent explostion, called a nova. The explosion of a massive star, called a supernova, can be billions of times as bright as the Sun , according to "Supernova," (World Book, Inc., 2005). Such a stellar explosion throws a large cloud of dust and gas into space, with the amount and composition of the material expelled varying depending on the type of supernova.
What is Moby's song "We are all made of stars" inspired by?
In 2002, music artist Moby released "We Are All Made of Stars," explaining during a press interview that his lyrics were inspired by quantum physics. "On a basic quantum level, all the matter in the universe is essentially made up of stardust," he said.
When were carbon, nitrogen and oxygen atoms created?
His statement sums up the fact that the carbon, nitrogen and oxygen atoms in our bodies, as well as atoms of all other heavy elements, were created in previous generations of stars over 4.5 billion years ago.
What is the material that is found in supernovae?
The material from a supernova eventually disperses throughout interstellar space. The oldest stars almost exclusively consisted of hydrogen and helium, with oxygen and the rest of the heavy elements in the universe later coming from supernova explosions, according to "Cosmic Collisions: The Hubble Atlas of Merging Galaxies," (Springer, 2009).
