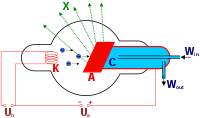
Each of the noble gasses glows in its own colour when exposed to high voltage; for example helium becomes pink, krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lamps. With rare exceptions, krypton is chemically inert.
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a colorless, dense, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, xenon can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, th…
Argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in the Earth's atmosphere, at 0.934%. It is more than twice as abundant as water vapor, 23 times as abundant …
What is the color of noble gas light?
The noble gases glow in distinctive colors when used inside gas-discharge lamps, such as " neon lights ". These lights are called after neon but often contain other gases and phosphors, which add various hues to the orange-red color of neon.
What is a noble gas?
She has taught science courses at the high school, college, and graduate levels. The elements in the last column or group of the periodic table share special properties. These elements are noble gases, sometimes called inert gases. Atoms belonging to the noble gas group have completely filled their outer electron shells.
Why do noble gases not take part in reactions?
The atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons. This is why the noble gases are inert and do not take part in chemical reactions. The table summarises the electronic configurations of elements in groups 1, 7 and 0.
What is the interaction between neon and noble gases?
The theoretical analysis of these interactions became tractable because the noble gases are monatomic and the atoms spherical, which means that the interaction between the atoms is independent of direction, or isotropic . Neon, like all noble gases, has a full valence shell.

Are all noble gases fluorescent?
Each noble gas glows a different color: for example, neon is reddish orange, like in neon signs. Argon glows blue, and krypton glows a whitish purple. Each of the noble gases (except radon) and their characteristic glow with an electrical discharge.
What color does each noble gas glow?
Neon lights were named for neon, a noble gas which gives off a popular orange light, but other gases and chemicals are used to produce other colors, such as hydrogen (red), helium (yellow), carbon dioxide (white), and mercury (blue).
Which noble gas glows green?
argonEach noble gas glows a specific color when electricity is passed through it, and the gases can be mixed to create other colors. Reds and oranges are produced by neon, while green is made when argon is put into tubes lined with a green fluorescent coating.
What noble gas glows white?
Krypton. Krypton emits a characteristic yellow-white light. This makes it useful for other colors; if the lamp's glass is colored, the light from the krypton will take on that new color.
Which gases produce a color glow?
The identity of the gas in the tube determines the color of the glow. Neon emits a red glow, helium produces pale yellow, and argon yields blue. Mercury vapor also emits blue light, and sodium vapor emits yellow. The majority of neon signs contain either neon gas or a mixture of neon and mercury vapor.
What noble gas glows yellow?
kryptonEach of the noble gasses glows in its own colour when exposed to high voltage; for example helium becomes pink, krypton glows yellow/green, xenon shines in lavender blue and argon in light blue.
What color does xenon glow?
blue glowXenon is used in certain specialised light sources. It produces a beautiful blue glow when excited by an electrical discharge.
Why do noble gasses light up?
In these lamps, a high voltage electrical discharge is passed through a tube of low-pressure neon gas. This electrical discharge can excite electrons in the neon atoms, causing them to jump from a low-lying and stable shell to a higher shell.
Is argon blue or purple?
Argon is element number 18 and has the atomic symbol Ar -- renamed in 1959 from its original atomic symbol, which was simply A. As you can see in the image above, argon gas produces a lovely bluish-purple colour when excited with electricity.
Does neon glow in the dark?
Most neon colors will glow in the dark underneath black lights. The most common colors to use are fluorescent orange, green, yellow and pink. White glows in a blueish looking color underneath black lights.
Why does argon glow?
First argon strikes the arc, this heats up and vaporizes mercury stuck to the sides. The arc then goes through mercury vapor, which creates UV light. The UV light excites the colored phosphor and you get your desired colored light.
What gas makes red neon?
Neon is red, helium is orange, argon is lavender, krypton is gray or green, mercury vapor is light blue, and xenon is gray or blue. Mixing gases and elements added to a neon light creates different hues.
What are the noble gas colors?
Each gas has a unique color that it emits.Colors of different noble gases when used in "neon" signs.Helium (pink)Neon (red-orange)Argon (blue)Krypton (pale green)Xenon (pale blue)
What Colour are the noble gases?
colourlessThe noble gases are colourless, odourless, tasteless, nonflammable gases.
What color does argon glow?
blueNeon and argon are most commonly used for lighting applications. Neon glows an orange-red, and argon glows blue. Mercury is added to the tubing to cause the gases to fluoresce. Additionally, different coatings made of color filtering chemicals called phosphors are added to tubes.
Why do noble gases show color?
It all comes down to how the excited electrons in each atom are able to find their way back home, and complete their outer shell. So we can trace both the stability of the noble gases, as well as the bright and lurid colours of the neon light back to the same quirk in quantum mechanics: Gilbert Lewis' rule of eight.
What are the properties of noble gases?
The properties of the noble gases can be well explained by modern theories of atomic structure: Their outer shell of valence electrons is considered to be "full", giving them little tendency to participate in chemical reactions, and it has been possible to prepare only a few hundred noble gas compounds.
What is the color of a noble gas?
Atomic number color: red=gas. v. t. e. The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity.
Why is helium used in balloons?
Since the Hindenburg disaster in 1937, helium has replaced hydrogen as a lifting gas in blimps and balloons due to its lightness and incombustibility , despite an 8.6% decrease in buoyancy. In many applications, the noble gases are used to provide an inert atmosphere.
Where does helium come from?
Helium is sourced from natural gas fields that have high concentrations of helium in the natural gas, using cryogenic gas separation techniques, and radon is usually isolated from the radioactive decay of dissolved radium, thorium, or uranium compounds.
What element was discovered in the air?
Along with Scottish scientist William Ramsay at University College, London, Lord Rayleigh theorized that the nitrogen extracted from air was mixed with another gas, leading to an experiment that successfully isolated a new element, argon, from the Greek word ἀργός ( argós, "idle" or "lazy").
How many electrons are in a neon?
Neon, like all noble gases, has a full valence shell. Noble gases have eight electrons in their outermost shell, except in the case of helium, which has two. The noble gases are colorless, odorless, tasteless, and nonflammable under standard conditions.
Which gases have low chemical reactivity?
The noble gases show extremely low chemical reactivity; consequently, only a few hundred noble gas compounds have been formed. Neutral compounds in which helium and neon are involved in chemical bonds have not been formed (although some helium-containing ions exist and there is some theoretical evidence for a few neutral helium-containing ones), while xenon, krypton, and argon have shown only minor reactivity. The reactivity follows the order Ne < He < Ar < Kr < Xe < Rn ≪ Og.
What is Xenox gas?
Xenox is a noble gas we encounter daily in the headlights of cars. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. The elements in the last column or group of the periodic table share special properties. ...
What is the difference between neon and helium?
Helium is so light it can escape the atmosphere and bleed away into space. Neon (Ne, atomic number 10) consists of a mix of three stable isotopes. The element is used to make signs and gas lasers and as a refrigerant. Neon, like helium, is inert under most conditions.
What is the atomic number of argon?
Argon (Ar, atomic number 18 ) in nature is a mixture of three stable isotopes. Argon is used in lasers and to provide an inert atmosphere for welding and chemicals, but it can form clathrates and has been known to form ions.
Which group of elements is non-reactive?
Atoms belonging to the noble gas group have completely filled their outer electron shells. Each element is non-reactive, has high ionization energy, electronegativity near zero, and a low boiling point. Moving down the group in the periodic table from top to bottom, the elements become more reactive. While helium and neon are practically inert and ...
Is argon a gas?
Argon is heavy enough that it doesn't readily escape Earth's gravity, so it is present in appreciable concentrations in the atmosphere. Krypton (Kr, atomic number 36) is a dense, colorless, inert gas. It's used in lasers and lamps. Xenon (Xe, atomic number 54) in nature consists of a mix of stable isotopes.
Is radon a noble gas?
Xenon is encountered in everyday life in xenon lamps such as strobe lamps and some vehicle headlamps. Radon (Rn, atomic number 86) is a heavy noble gas. All of its isotopes are radioactive. Although colorless under ordinary conditions, radon is phosphorescent as a liquid, glowing yellow and then red.
Is helium a gas?
Helium (He, atomic number 2) is an extremely light, inert gas at room temperature and pressure. The liquid form of the element is the only liquid known to man that cannot be solidified, no matter how low the temperature drops. Helium is so light it can escape the atmosphere and bleed away into space.
History
Noble gases contain helium, neon, argon, krypton, Xenon, and radon. None of the noble gases was known when Mendeleev proposed his periodic table. In 1892, The English Scientist Willaim Ramsay became interested in the discovery.
Introduction of Noble gases
What do party balloons, neon signs, and certain light bulbs have in common they are all filled with a noble gas in this.
Xenon fluorine compounds
Xenon forms three binary fluorides XeF 2, XeF 4 and XeF 6 by using the direct reaction of elements under appropriate experimental conditions.
Why do noble gases glow?
Well scientists believe that the Noble gases glow when a chemical current is passed through them because the outer energy levels are full they give out another energy level which results in the "neon" glow.
What column is the noble gas in?
The Noble Gases are column 18 of the periodic table.
What is the atomic number of neon glasses?
Neon- Neon has an atomic number of 10 and an atomic weight of 20.1797 Neon has the most intense glow of all the other noble glasses.
Is radon a gas?
When the temperature is below freezing then Radon begins to glow a yellow color. Radon gas can be dangerous for humans to breath in and it used to be in homes which has now become a safety hazard.
What are the properties of noble gases?
The main properties of the noble gases include: they have low densities. they are inert, so they are not flammable. Many uses of the noble gases are linked to one or more of these properties.
Why are noble gases inert?
Explaining the inertness of noble gases. When elements react, their atoms complete their outer shells by losing, gaining, or sharing electrons. The atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons. This is why the noble gases are inert and do not take part in chemical reactions.
Overview
Applications
Noble gases have very low boiling and melting points, which makes them useful as cryogenic refrigerants. In particular, liquid helium, which boils at 4.2 K (−268.95 °C; −452.11 °F), is used for superconducting magnets, such as those needed in nuclear magnetic resonance imaging and nuclear magnetic resonance. Liquid neon, although it does not reach temperatures as low as liquid helium, a…
History
Noble gas is translated from the German noun Edelgas, first used in 1898 by Hugo Erdmann to indicate their extremely low level of reactivity. The name makes an analogy to the term "noble metals", which also have low reactivity. The noble gases have also been referred to as inert gases, but this label is deprecated as many noble gas compounds are now known. Rare gases is anot…
Physical and atomic properties
The noble gases have weak interatomic force, and consequently have very low melting and boiling points. They are all monatomic gases under standard conditions, including the elements with larger atomic masses than many normally solid elements. Helium has several unique qualities when compared with other elements: its boiling point at 1 atm is lower than those of any other …
Chemical properties
The noble gases are colorless, odorless, tasteless, and nonflammable under standard conditions. They were once labeled group 0 in the periodic table because it was believed they had a valence of zero, meaning their atoms cannot combine with those of other elements to form compounds. However, it was later discovered some do indeed form compounds, causing this label to fall into dis…
Occurrence and production
The abundances of the noble gases in the universe decrease as their atomic numbers increase. Helium is the most common element in the universe after hydrogen, with a mass fraction of about 24%. Most of the helium in the universe was formed during Big Bang nucleosynthesis, but the amount of helium is steadily increasing due to the fusion of hydrogen in stellar nucleosynthesis (and, to a very slight degree, the alpha decay of heavy elements). Abundances on Earth follow diff…
Discharge color
The color of gas discharge emission depends on several factors, including the following:
• discharge parameters (local value of current density and electric field, temperature, etc. – note the color variation along the discharge in the top row);
• gas purity (even small fraction of certain gases can affect color);
See also
• Noble gas (data page), for extended tables of physical properties.
• Noble metal, for metals that are resistant to corrosion or oxidation.
• Inert gas, for any gas that is not reactive under normal circumstances.