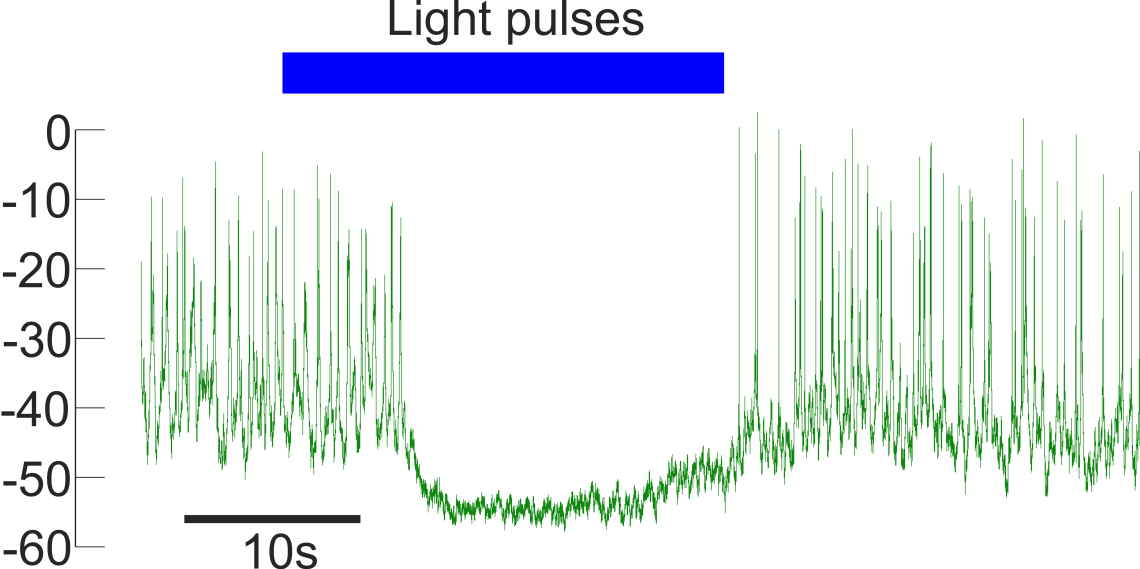
What stimulates glucagon release from alpha cells?
AA and catecholamines are powerful physiological stimulators of glucagon release from alpha cells, and glucose inhibits these actions. The signaling pathways that operate here are not well understood but may involve steps similar to those described for beta cells, including nucleotide-controlled K+ channels and L-type Ca 2+ channels.
How does glucagon regulate blood sugar levels?
Your body normally carefully regulates your blood glucose (sugar) primarily with the hormones glucagon and insulin. When your blood glucose levels trend lower or fall too low ( hypoglycemia ), your pancreas releases more glucagon. Glucagon helps blood glucose levels rise back up in multiple ways, including:
Where is glucagon synthesized in the pancreas?
Glucagon is synthesized in and secreted from A-cells of pancreatic islets. Normally, these cells constitute approximately 15% to 20% of the total islet cell mass.
What is glucagon?
Glucagon Physiology - Endotext - NCBI Bookshelf Glucagon is a peptide hormone secreted from the alpha cells of the pancreatic islets of Langerhans.

How is glucagon secreted?
Glucagon is secreted in response to hypoglycemia, prolonged fasting, exercise and protein-rich meals (10) . Glucagon release is regulated through endocrine and paracrine pathways; by nutritional substances; and by the autonomic nervous system (11). Glucagon secretion occurs as exocytosis of stored peptide vesicles initiated by secretory stimuli of the alpha cell. Stimulatory regulators of glucagon release include hypoglycemia, amino acids and the gut hormone glucose-dependent insulinotropic peptide (GIP), whereas hyperglycemia and GLP-1 inhibit glucagon release. Additionally, glucagon release is inhibited in a paracrine fashion by factors like somatostatin, insulin, zinc and possibly amylin. Glucagon may regulate its own secretion indirectly via stimulatory effect on beta cells to secrete insulin (12,13). In contrast to glucose, non-glucose regulators of glucagon secretion seem to mediate their action through changes in cAMP levels rather than through the calcium-dependent pathway outlined below (14,15).
What is the mechanism of glucagon release?
Glucagon secretion occurs as exocytosis of stored peptide vesicles initiated by secretory stimuli of the alpha cell. Stimulatory regulators of glucagon release include hypoglycemia, amino acids and the gut hormone glucose-dependent insulinotropic peptide (GIP), whereas hyperglycemia and GLP-1 inhibit glucagon release.
How does glucagon affect glucose?
Glucagon controls plasma glucose concentrations during fasting, exercise and hypoglycemia by increasing hepatic glucose output to the circulation. Specifically, glucagon promotes hepatic conversion of glycogen to glucose (glycogenolysis), stimulates de novoglucose synthesis (gluconeogenesis), and inhibits glucose breakdown (glycolysis) and glycogen formation (glycogenesis) (Fig. 5) (26). Hepatic glucose production is rapidly enhanced in response to a physiological rise in glucagon; achieved through stimulation of glycogenolysis with minor acute changes in gluconeogenesis (27,28). This ability of glucagon is critical in the life-saving counterregulatory response to severe hypoglycemia. Additionally, it is a key factor in providing adequate circulating glucose for brain function and for working muscle during exercise (28). During prolonged fasting, glycogen stores are depleted, and gluconeogenesis takes over (29). The hyperglycemic property of glucagon is enhanced when hepatic glycogen levels are high and diminished when hepatic glycogen levels are low in conditions of fasting or liver diseases like cirrhosis (12).
What is the role of glucagon in the body?
Hypoglycemia is physiologically the most potent secretory stimulus and the best known action of glucagon is to stimulate glucose production in the liver and thereby to maintain adequate plasma glucose concentrations. However, glucagon is also involved in hepatic lipid and amino acid metabolism and may increase resting energy expenditure. Based on satiety-inducing and food intake-lowering effects of exogenous glucagon, a role for glucagon in the regulation of appetite has also been proposed. This chapter provides an overview of the structure, secretion, degradation and elimination of glucagon, and reviews the actions of glucagon including its role in glucose metabolism and its effects on lipolysis, ketogenesis, energy expenditure, appetite and food intake. Finally, the role of glucagon in the pathophysiology of diabetes, obesity and hepatic steatosis is discussed and emerging glucagon-based therapies for these conditions are outlined. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
What enzyme is used to make proglucagon?
In the pancreas proglucagon is processed into glucagon, glicentin-related pancreatic polypeptide (GRPP), intervening peptide 1 (IP1), and major proglucagon fragment (MPGF) by the processing enzyme prohormone convertase 2 (PC2) . In the intestine and in the brain proglucagon is processed by prohormone convertase 1/3 (PC1/3) into glucagon-like peptide 1 (GLP-1), glucagon-like peptide 2 (GLP-2), oxyntomodulin, intervening peptide 2 (IP2), and glicentin.
Where is the glucagon receptor located?
The glucagon receptor is a seven transmembrane G protein-coupled receptor (Fig. 4) predominantly expressed in the liver, but also found in varying amounts in the kidneys, heart (controversial), adrenal glands, adipose tissue (controversial), gastrointestinal tract, and pancreas (21).
Which cell is the most potent regulator of glucagon secretion?
Regulation of Glucagon Secretion by Glucose. The most potent regulator of glucagon secretion is circulating glucose. Hypoglycemia stimulates the pancreatic alpha cell to release glucagon and hyperglycemia inhibits glucagon secretion (Fig. 2) (11).
Summary
In the pancreatic islet, serotonin is an autocrine signal increasing beta cell mass during metabolic challenges such as those associated with pregnancy or high-fat diet. It is still unclear whether serotonin is relevant for regular islet physiology and hormone secretion.
Introduction
Serotonin was proposed as a paracrine signal in the pancreatic islet over 40 years ago ( Lundquist et al., 1971, Marco et al., 1977, Pontiroli et al., 1978 ). Serotonin is co-released with insulin ( Ekholm et al., 1971, Jaim-Etcheverry and Zieher, 1968, Richmond et al., 1996 ).
Results
We found that human islets contained at least three times more serotonin-positive cells than mouse islets (5-hydroxytryptamine [5HT]) ( Figures 1 A and 1B ). Serotonin immunostaining varied between human pancreases.
Discussion
The results presented here firmly establish that serotonin is produced and released by beta cells in islets from non-diabetic, non-pregnant individuals. We show that human alpha cells lose the ability to respond appropriately to changes in glucose when islet serotonin levels are manipulated pharmacologically.
Experimental Procedures
We obtained human pancreatic islets from the Integrated Islet Distribution Program (IIDP) ( Table S1) and human pancreatic tissue samples (from the head of the pancreas) from the Human Islet Cell Processing Facility at the Diabetes Research Institute, University of Miami.
Author Contributions
J.A., J.M., and D.M. performed experiments with biosensor cells, hormone assays and ELISAs. J.A. performed immunohistochemistry, quantitative real-time PCR, and cAMP measurements. A.N.P. and V.S. performed in situ hybridization. J.A., A.T., and A.C. conducted in vivo studies. J.A., P.-O.B., and A.C.
Acknowledgments
We thank Juan Ricardo Ortiz Meneses and Maikel Rivero for help with immunohistochemistry, Kristin Perez and Simón Caicedo for data analyses, and Kevin Johnson for histological work.
Where is glucagon produced?
Glucagon is a 29–amino acid peptide produced in A cells of the pancreas.
How do amino acids release glucagon?
This effect is glucose dependent and is best observed at low glucose concentrations. The stimulatory effect of amino acids on glucagon secretion appears to be mediated, at least in part, by a direct interaction with the alpha cell, as indicated by studies measuring glucagon release from purified alpha-cell preparations. Current evidence suggests that the release of glucagon is triggered by the binding of amino acids to membrane receptors , a process that is Ca2+ dependent. Changes in second messengers, such as diacylglycerol, Ca2+, and cAMP, may also be involved.
What is the purpose of stimulating glucagon release during ingestion of a mixed meal?
Stimulation of glucagon release during ingestion of a mixed meal—presumably the result of amino acids from the digested protein in the meal—would act to balance the actions of concomitantly released insulin ( e.g., suppression of hepatic glucose release) to prevent postprandial hypoglycemia. View chapter Purchase book.
How does insulin affect the pancreas?
First, insulin activates the PI3K signaling pathway, which modifies the activity of K ATP channels and inhibits glucagon secretion . Second, insulin may translocate A-type gamma aminobutyric acid (GABA) receptors to the cell membrane of the α-cells; GABA released by the β-cells (together with insulin) binds to GABA receptors on the plasma membrane of the α-cells, which triggers changes in the cell membrane potentials and consequently suppresses glucagon secretion. Finally, zinc released together with insulin may also inhibit glucagon secretion through alteration of ion channel activity. Hyperglucagonemia seen in type 1 diabetes partly results from the lack of the inhibitory effects of insulin on the α-cells due to β-cell destruction.
How does glucose affect glucagon secretion?
Multiple paracrine and neural inputs modulate glucagon secretion in response to glucose and other nutrients. The islet α-cells are equipped with multiple channels that regulate cell membrane potentials or generate action potentials. The α-cells and β-cells have opposite Ca + signaling patterns in response to glucose. At low glucose concentration, the cytosolic ATP/ADP ratio is low; K ATP channels demonstrate a moderate activity, which situates the α-cells to a membrane potential that allows the opening of voltage-dependent T- and N-type Ca 2 + channels. The resulting increased intracellular calcium concentrations, in turn, stimulate exocytosis and glucagon secretion. On the contrary, high glucose blocks K ATP channels, which depolarizes the α-cells to a membrane potential range that suppresses the voltage-dependent Ca 2 + channels. Consequently, Ca 2 + signaling and glucagon release are blocked. However, several studies have suggested that the effect of low glucose directly on the α-cells is not a major mediator of the glucagon response to hypoglycemia.
Why is hyperglucagonemia seen in type 1 diabetes?
Hyperglucagonemia seen in type 1 diabetes partly results from the lack of the inhibitory effects of insulin on the α-cells due to β-cell destruction . Somatostatin is a potent regulator of glucagon secretion.
What are the mediators of glucagon secretion?
The control of glucagon secretion is multifactorial and involves direct effects of nutrients on the α-cell stimulus-secretion coupling as well as paracrine regulation by insulin, somatostatin, and possibly, other mediators such as zinc, γ-amino-butyric acid (GABA), or glutamate ( Gromada et al., 2007, 2018; Walker et al., 2011 ). Glucagon secretion is also regulated by circulating hormones and the autonomic nervous system (reviews in Thorens, 2011; Holst et al., 2011 ).
Why is glucagon not producing?
One proposed theory is that because of the dysfunctional (or non-existant) insulin production, glucagon production isn’t able to accurately determine when it should or should n’t increase its production.
What hormone is secreted by alpha cells?
Glucagon, on the other hand, is a hormone secreted by alpha-cells, also produced by your pancreas. This hormone tells your liver to release glycogen — which is basically stored sugar.
How does insulin work?
This class of drugs works entirely by reducing the amount of glucose produced by your liver. This inevitably makes you more sensitive to insulin. It also reduces the amount of sugar you absorb from the food you eat and reduces your appetite.
What type of cell dysfunction is found in type 1 diabetes?
In a person with type 1 diabetes and type 2 diabetes, there is alpha-cell dysfunction.
