What should the overall charge of a compound be?
The overall or net charge of any ionic compound must equal zero. Although ions themselves are either positive (cation) or negative (anion) by definition, the only way they can bond together and form a compound is by seeking an opposite charge. An ionic compound in chemistry refers to any chemical compound in which ions are held together, or ...
Does a compound have a charge?
All compounds can be taken up as molecules and a compound is an actual matter in it complete shape. A compound may have a positive or negative charge. The most commonly used compound is H 2 O. Compounds are classified into molecular compounds, ionic compounds, intermetallic compounds, and complexes.
Do compounds have a neutral charge?
Ionic compounds are electrically neutral because the charges of the cations and anions that make up the compound cancel each other out. In the case of salt for example, sodium has a charge of positive one, and chloride has a charge of negative one. Together, they neutralize the compound.
How to find charge on compound?
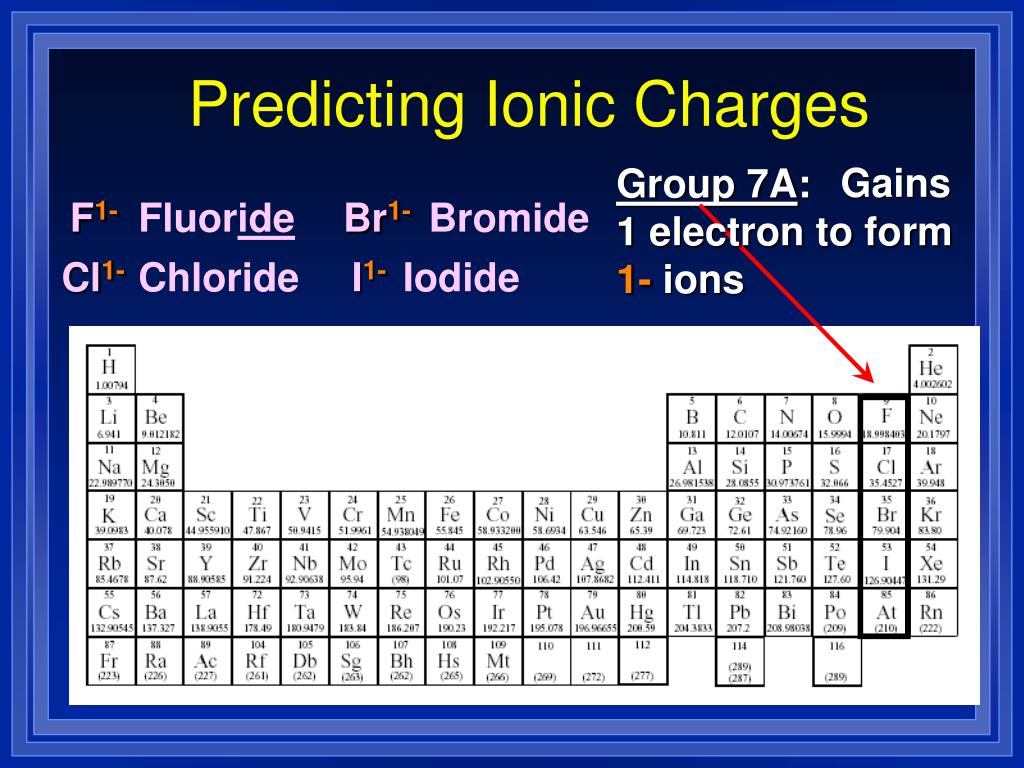
For a single atom, the charge is the number of protons minus the number of electrons. Find the charge by balancing charge in a compound. Table of common element charges
Do compounds have no charge?
Since an ionic compound consists of equal number of positive and negative ions, the overall charge of an ionic compound is zero.
Does a compound have a positive or negative charge?
So far, we have discussed elements and compounds that are electrically neutral. They have the same number of electrons as protons, so the negative charges of the electrons is balanced by the positive charges of the protons. However, this is not always the case....Answers.NameFormula and ChargetriiodideI 3 −8 more rows•Nov 14, 2019
What is the charge of all compounds?
zeroAny ionic compound will have a net charge of zero. Another way of saying this is that cations and anions must always combine in such a way so that their charges cancel. The number of cations and anions in the formula should be written as the lowest possible integer value.
How do you know if a compound has a charge?
0:543:12How to Identify the Charge of an Ion : Chemistry Lessons - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo if it's an ion it has eight already which means that it has one more electron than it has aMoreSo if it's an ion it has eight already which means that it has one more electron than it has a neutral charge. So a chloride a chlorine ion or chloride is going to have a charge of negative one.
Are ionic compounds negative or positive?
Ionic compounds are neutral compounds made up of positively charged ions called cations and negatively charged ions called anions. For binary ionic compounds (ionic compounds that contain only two types of elements), the compounds are named by writing the name of the cation first followed by the name of the anion.
What has no charge at all?
neutron, neutral subatomic particle that is a constituent of every atomic nucleus except ordinary hydrogen. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1,839 times greater than that of the electron.
What elements have a charge?
Table of Common Element ChargesNumberElementCharge10neon011sodium1+12magnesium2+13aluminum3+88 more rows•Dec 23, 2018
Does NaCl have a charge?
Ionic compounds are always neutral. The formula for sodium chloride, therefore, is NaCl since Na loses one electron and has a +1 charge and Cl gains one electron giving it a -1 charge. Some atoms may, however, gain or lose more than one electron.
Are ionic compounds electrically neutral?
In every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. Thus, ionic compounds are electrically neutral overall, even though they contain positive and negative ions.
Can covalent compounds have a charge?
The Covalent Bond A single covalent bond is created when two atoms share a pair of electrons. There is no net charge on either atom; the attractive force is produced by interaction of the electron pair with the nuclei of both atoms.
How do you know if a compound is neutral?
A solution's pH will be a number between 0 and 14. A solution with a pH of 7 is classified as neutral. If the pH is lower than 7, the solution is acidic. When pH is higher than 7, the solution is basic.
How do you find a charge?
0:081:46Calculating the Charge of an Atom - YouTubeYouTubeStart of suggested clipEnd of suggested clipBy comparing the number of protons to the number of electrons. We can determine whether the chargeMoreBy comparing the number of protons to the number of electrons. We can determine whether the charge of an atom is positive or negative and also can calculate the total charge of the atom.
What is the overall charge of an ionic compound?
The overall or net charge of any ionic compound must equal zero. Although ions themselves are either positive (cation) or negative (anion) by definition, the only way they can bond together and form a compound is by seeking an opposite charge. An ionic compound in chemistry refers to any chemical compound in which ions are held together, or bound, ...
Why does each ion hold a neutral charge?
The structure of each ion is different than an atom of the same element (Na or Cl) that holds a neutral charge due to an equal number of protons and electrons. It is only once each atom becomes an ion that is it able to bond with the other atom and create an ionic compound. ADVERTISEMENT.
What is an ionic compound?
An ionic compound in chemistry refers to any chemical compound in which ions are held together, or bound, through ionic bonds. Ionic bonds, by definition, are electrostatic attractions between two ions with an opposite charge. In other words, an ionic bond can only form between a cation and an anion. Although there is no limit to the number of ions ...
Which compound has an equal number of cations and anions?
Therefore, there must be an equal number of cations and anions within the completed compound. One popular, and common, example of an ionic compound which displays this principle is table salt or sodium chloride, which has the chemical formula NaCl.
Can an ionic bond be formed between a cation and an anion?
In other words, an ionic bond can only form between a cation and an anion. Although there is no limit to the number of ions contained in an ionic compound, in order for the compound to remain whole, the net charge needs to equal zero.
What is the charge of an atom?
The charge on an atom is related to its valence electrons or oxidation state. An atom of an element is most stable when its outer electron shell is completely filled or half-filled. The most common charges are based on maximum stability for the atom. However, other charges are possible.
Does hydrogen have a charge?
For example, hydrogen sometimes has a charge of zero or (less commonly) -1. Although noble gas atoms almost always carry a charge of zero, these elements do form compounds, which means they can gain or lose electrons and carry a charge.