What is an E2 reaction?
The reaction involves two compounds; the alkyl halide and a base. Hence it is known as a bimolecular reaction. E2 reactions occur in the presence of a strong base. The most common example for E2 reactions is dehydrohalogenation.
How do you stabilize carbocations in E1 reactions?
In the second step (deprotonation), the carbocation is stabilized by removal of a hydrogen atom as a proton. Usually, E1 reactions take place with tertiary alkyl halides.
What is the difference between E1 and E2 carbons?
E1 has no need for an antiperiplanar orientation, but E2 does. E1 has a carbocation intermediate, which allows for 1,2-hydride shifts or 1,2-alkyl shifts, but E2 does not have or allow either. WHEN YOU HAVE A POOR LEAVING GROUP
Why is the carbon in red in E1 mechanism?
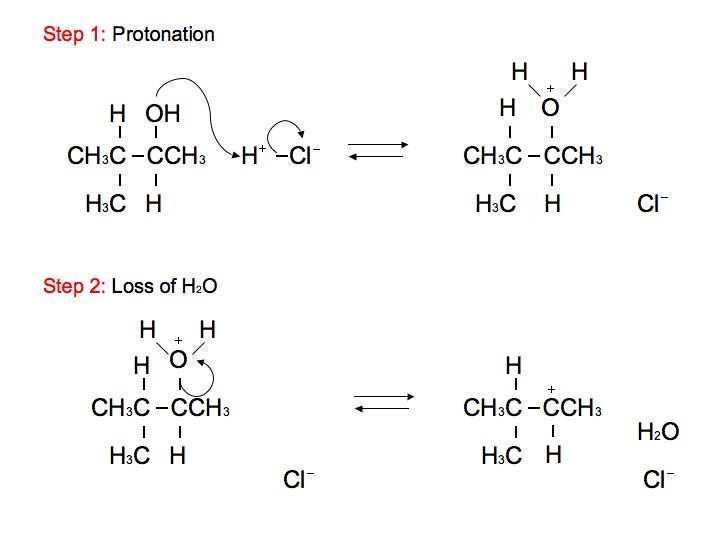
Now, the reason why this step happens first when you have an alcohol is it forms a better leaving group for your E1 mechanism. Water is a much better leaving group than the hydroxide anion. So next, the electrons in this bond come off onto the oxygen, and water leaves. So when we do that, we take a bond away from this carbon, the carbon in red.
Does E2 form a carbocation?
Comparing E1 and E2 mechanisms 3) The alkyl halide: primary alkyl halides have the only structure useful in distinguishing between the E2 and E1 pathways. Since primary carbocations do not form, only the E2 mechanism is possible.
Do E2 reactions have intermediates?
E2 Definition. The E2 reaction - A Nucleophilic Elimination reaction in which the Rate Determining Step involves 2 components. -E2 reactions are bimolecular, with simultaneous bond-making and bond breaking steps. -E2 reactions do not proceed through an intermediate.
What is the main difference between E1 and E2 reactions?
The key differences between the E2 and E1 mechanism are: 1) E2 is a concerted mechanism where all the bonds are broken and formed in a single step. The E1, on the other hand, is a stepwise mechanism.
What characteristics describe an E2 reaction?
E2 indicates an elimination, bimolecular reaction, where rate = k [B][R-LG]. In an E2 reaction, the reaction transforms 2 sp3 C atoms into sp2 C atoms. This moves the substituents further apart decreasing any steric interactions. So more highly substituted systems undergo E2 eliminations more rapidly.
Does E1 have a carbocation?
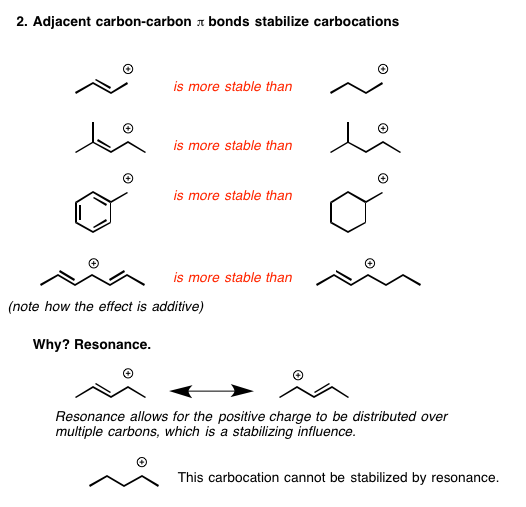
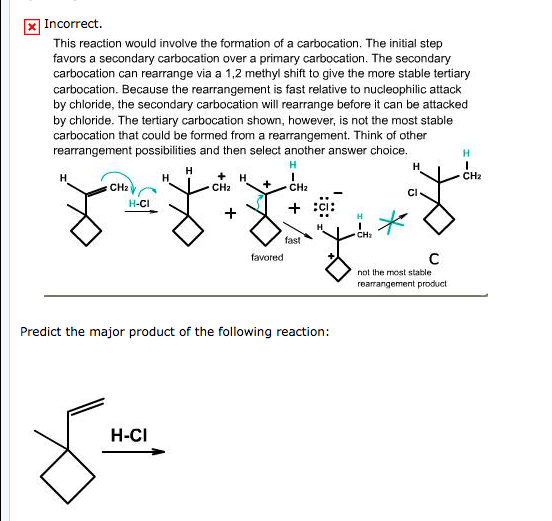
Since carbocation intermediates are formed during an E1, there is always the possibility of rearrangements (e.g. 1,2-hydride or 1,2-alkyl shifts) to generate a more stable carbocation.
What is a carbocation intermediate?
A carbocation is an organic molecule, an intermediate that has a carbon atom bearing a positive charge and three bonds instead of four.
Which is more stable E1 or E2?
Mechanistically, E2 reactions are concerted (and occur faster), whereas E1 reactions are stepwise (and occur slower and at a higher energy cost, generally). Due to E1's mechanistic behavior, carbocation rearrangements can occur in the intermediate, such that the positive charge is relocated on the most stable carbon.
Why does E2 require a strong base?
The rate of the E2 reaction depends on both substrate and base, since the rate-determining step is bimolecular (concerted). A strong base is generally required, one that will allow for displacement of a polar leaving group.
Does E2 have inversion?
SN2 and E2 Reactions Rate and stereochemical experiments show that the SN2 mechanism proceeds through nucleophilic backside attack on the α-carbon with inversion of stereochemical configuration. Similar experiments with E2 reactions reveal the elimination of a β-hydrogen and the formation of a double bond.
How would you distinguish between elimination E1 and E2 reactions?
The key difference between E1 and E2 reactions is that E1 reactions have unimolecular elimination mechanism whereas E2 reactions have bimolecular elimination mechanism.
What happens E2 reaction?
E2, bimolecular elimination, was proposed in the 1920s by British chemist Christopher Kelk Ingold. Unlike E1 reactions, E2 reactions remove two subsituents with the addition of a strong base, resulting in an alkene.
What factors affect E2 reactions?
Factors affecting E2 reactionsAs the strength of the base is increased, E2 reactions are accelerated.E2 reactions often involve strong nonpolar bases, such as OH.OH is often used to carry out E2 reactions since it is a nonpolar base.A stronger leaving group results in faster E2 reactions.More items...
What is the mechanism of E2?
The reaction mechanisms of E2 reactions are known as bimolecular eliminations. The E2 reaction mechanism is a single step elimination reaction with a single transition state. Therefore, the chemical bond breakdown and formation occurs in the same step. This type of reactions is often found in primary alkyl halides.
What is an E1 reaction?
E1 reactions are a type of two-step elimination reactions found in organic chemistry. In these elimination reactions, substituents in organic compounds are eliminated or removed. The reaction mechanisms of E1 reactions are known as unimolecular eliminations. E1 reactions are two-step reactions, which means, an E1 reaction occurs through two steps ...
What is the difference between E1 and E2?
The key difference between E1 and E2 reactions is that E1 reactions have unimolecular elimination mechanism whereas E2 reactions have bimolecular elimination mechanism. In organic chemistry, elimination reactions are a special type of chemical reactions in which substituents are removed (eliminated) from organic compounds.
Why are bulky alkyl halides unable to undergo E2 reactions?
There are two reasons for this; bulky alkyl halides (highly substituted) are unable to undergo E2 reactions, and highly substituted carbocations are more stable than primary or secondary carbocations. In E1 reactions, the carbocation formation is the slowest step. Therefore, it is the rate-determining step pf E1 reactions, ...
What is the second step of carbocation?
In the second step (deprotonation), the carbocation is stabilized by removal of a hydrogen atom as a proton. Usually, E1 reactions take place with tertiary alkyl halides. But sometimes, secondary alkyl halide also undergoes this type of elimination reactions.
What conditions are used for E1 reactions?
E1 reactions usually take place in the complete absence of bases or the presence of weak bases. Acidic conditions and high temperatures are preferred for successful E1 reaction. And also, E1 reactions include carbocation rearrangement steps.
How does E1 react?
E1 reactions are two-step reactions, which means, an E1 reaction occurs through two steps named as ionization and deprotonation. In the ionization process, a carbocation is formed due to the removal of a substituent. In the second step (deprotonation), the carbocation is stabilized by removal of a hydrogen atom as a proton.
Which solvents favor SN1 and E1 reactions?
Polar, protic solvents favor SN1 and E1 reactions. The polar and protic properties of the solvent stabilize the carbocation and solvate the leaving group. This may seem at odds with SN2 's increased reactivity in polar, aprotic solvents. Protic solvents blunt the nucleophile.
Which bond is the antibond of the carbocation?
The antibond points only directly opposite the C-LG bond. Since electrons can only come in from one direction, the bimolecular reactions are stereospecific. On the other hand, the carbocation has no σ* C-LG antibond. Instead, the carbocation loses its original shape to become planar.
What solvents slow down SN2?
Protic solvents blunt the nucleophile. Since nucleophilicity is a key part of the reaction's rate- limiting transition state, protic solvents slow down SN2 reactions. For the unimolecular reactions, however, the rate-limiting step does not involve the solvent's nucleophilicity.
Do alkanes donate electrons?
Alkanes are slightly electron donating. This explains why SN1 and E1 reactions need a secondary or tertiary α -carbon. The carbocation-like transition state of the tertiary α -carbon is more stable than that of the secondary α -carbon, and so on.
Does E1 have Saytzeff's rule?
The E1 reaction undergoes a similar effect, though it does retain Saytzeff's rule. Saytzeff's rule is founded on energetic stability, and thus is not affected by the random geometry introduced by the carbocation.
Is SN2 a concerted reaction?
SN2 and E2 reactions have very stereospecific properties. They owe this to their concerted mechanisms. SN1 and E1 reactions are not concerted. They share a common carbocation intermediate. The carbocation intermediate ruins the stereospecificity of the reaction.
What happens if a C is sp2 hybridized?
If a C is sp2 hybridized, that doesn’ t mean that the C has to be double bonded to another C, sp2 hybridization requires only that there be three other atoms directly attached to the C. This is the case with the methyl cation, CH3+. Comment on Ernest Zinck's post “If a C is sp2 hybridized,...”.
What happens if you shift the methyl group?
If you shift the methyl group, you get a 2° 1,2-dimethhyl propyl cation. You would be converting one 2° carbocation to a different 2° carbocation. This is not favorable, as there is no difference in energy between in the two carbocations.