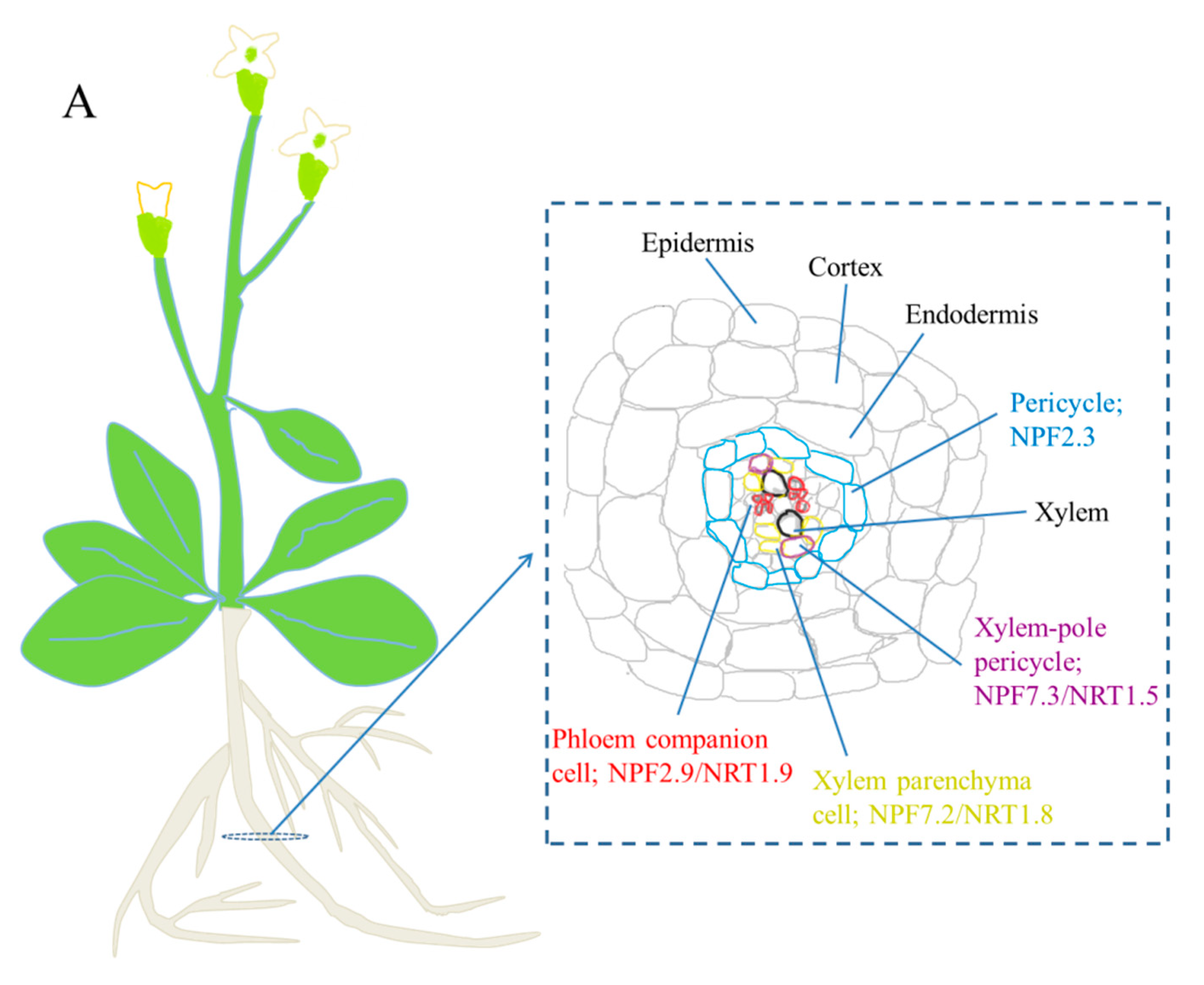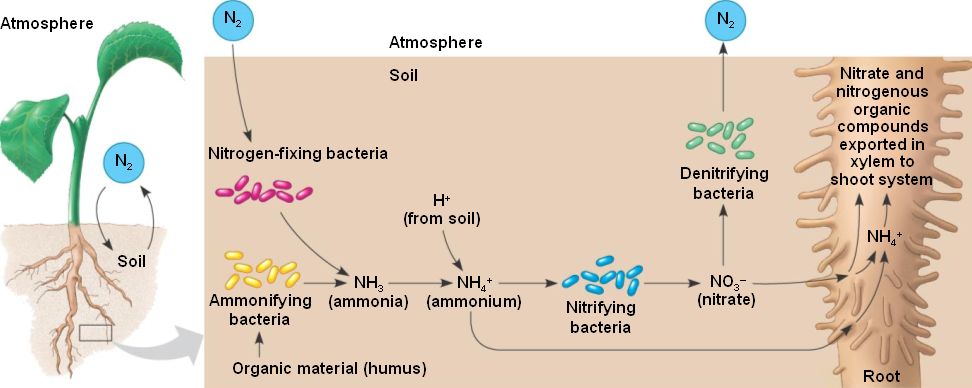
How do plants absorb nitrate from soil?
Plants can absorb nitrate or ammonium from the soil via their root hairs. If nitrate is absorbed, it is first reduced to nitrite ions and then ammonium ions for incorporation into amino acids, nucleic acids, and chlorophyll.
How do plants get nitrogen from the soil?
Most nitrogen obtained by terrestrial animals can be traced back to the eating of plants at some stage of the food chain. Plants can absorb nitrate or ammonium from the soil via their root hairs. If nitrate is absorbed, it is first reduced to nitrite ions and then ammonium ions for incorporation into amino acids, nucleic acids, and chlorophyll.
Are nitrites used by plants and animals?
However nitrites are not usable by plants and animals directly, some bacteria can change nitrites into nitrates, with the help of oxygen, and in the process getting energy out of it. The nitrates then can be used as nitrogen for the plants.
Do Plants take up nitrite (NO2–)?
It doesn't look like plants take up nitrite (NO2–), only ammonium (NH4+) and nitrate (NO3–). Interestingly, however, nitrate taken up, in some cases, is reduced to nitrite for use in some pathways. You’ve done what you can to cut back your spending. You brew coffee at home, you don’t walk into Target and you refuse to order avocado toast.

Why do plants use nitrates?
Nitrates are nitrate salts which plants cannot take in and which are actually hard to find in natural waters because they are highly soluble. )
How do plants absorb nitrogen?
Plants absorb nitrogen through their roots, mostly in the form of nitrate ( N O 3 − ). This is part of the Nitrogen cycle, which is a crucial part of the biosphere.
How does nitrogen get into the soil?
They have to make do with nitrogen that comes from decaying organic matter, or from ammonium and other nitrogen-containing compounds that are converted into nitrate by nitrifying bacteria in the soil.
Why is nitrate more stable than ammonium?
There are some potential reasons for existence of both. Nitrate is more stable as ammonium easily loses a proton and the resulting ammonia gas (NH3) escapes . In fact, some plants seem to promote the bacterial nitrification (NH4+ to NO3-) process, and the escape of NH3 may give an explanation of the fitness advantage of such an ability. On the other hand, NH4+ tends to stick to earth particles whereas NO3- is highly mobile and therefore easier for the plant to uptake. And not all plants favor nitrification, some inhibit it.
How is ammonia converted to nitrates?
In water through their roots. Ammonia is converted to nitrates by nitrifying bacteria in the soil. Plants absorb these nitrates and use them to build up proteins. Without nitrates, the amount of chlorophyll in leaves is reduced.
Why are plants dependent on nitrogen?
Plants are dependent on external fixed nitrogen because that's how they found it. The world was swarming with cyanobacteria long before plants got there. Cyanobacteria produce fixed nitrogen with an enzyme called nitrogenase as part of their metabolism; they evolved it completely independently of plants.
Why are there different kinds of plants?
The reason why there are different kinds of plants may also come from population dynamics. A population with both NH4+ uptakers and NO3- uptakers may prosper better than a plant community in which everybody prefers the same form of nitrogen. Please see the paper by S. Boudsocq and co-workers in American Naturalist 180: 60–69, 2012. DOI: 10.1086/665997.
Why is nitrification important for plants?
The process of nitrification is important for plants and will make sure the plants always have enough nitrogen available if they need some.
How Do Plants Get Nitrogen?
The presence of all the required chemical compounds in a fixed and required amount will lead to the healthy development of a plant. Among all the important chemical compounds required by the plant, nitrogen is one of them and there are actually only two ways how plants get nitrogen:
How to fix nitrogen deficiency in soil?
When there is a nitrogen deficiency in the soil, you have two options to fix the nitrogen in the ground.
Why is nitrogen a deficiency?
Although Nitrogen is one of the most abundant elements in the earth but still in the plants the common deficiency problem is related to nitrogen, this is because nitrogen from the atmosphere or from the earth’s crust is not directly available for the plants. This deficiency can be fixed by adding fertilizer to the soil. The fertilizer can be absorbed by the plant and used as nitrogen.
What is the first form of nitrogen produced by mineralization?
The first form of nitrogen produced by this process is ammonia, NH3. This ammonia will react with water to form ammonium NH4, which will be available for plants.
What is phase 2 of a plant?
Phase 2: Mineralization. This phase takes place in the soil of the plants. Nitrogen moves from organic matirials, such as plant to an inorganic form of nitrogen that plants can use. Decomposed plants are being used for nitrogen in other plants as well as animal materials that are left on the soil.
Why is nitrogen immobilized?
When the bacteria in the ground would use up all the nitrogen, the plants would soon be deficient in nitrogen. That is why immobilization ties up nitrogen in microorganisms and helps to maintain balance the amount of nitrogen in the soils by tying it up or immobilizing the nitrogen in microorganisms.
Why do plants use nitrates?
Nitrates are nitrate salts which plants cannot take in and which are actually hard to find in natural waters because they are highly soluble. )
How do plants absorb nitrogen?
Plants absorb nitrogen through their roots, mostly in the form of nitrate ( N O 3 − ). This is part of the Nitrogen cycle, which is a crucial part of the biosphere.
How does nitrogen get into the soil?
They have to make do with nitrogen that comes from decaying organic matter, or from ammonium and other nitrogen-containing compounds that are converted into nitrate by nitrifying bacteria in the soil.
Why is nitrate more stable than ammonium?
There are some potential reasons for existence of both. Nitrate is more stable as ammonium easily loses a proton and the resulting ammonia gas (NH3) escapes . In fact, some plants seem to promote the bacterial nitrification (NH4+ to NO3-) process, and the escape of NH3 may give an explanation of the fitness advantage of such an ability. On the other hand, NH4+ tends to stick to earth particles whereas NO3- is highly mobile and therefore easier for the plant to uptake. And not all plants favor nitrification, some inhibit it.
How is ammonia converted to nitrates?
In water through their roots. Ammonia is converted to nitrates by nitrifying bacteria in the soil. Plants absorb these nitrates and use them to build up proteins. Without nitrates, the amount of chlorophyll in leaves is reduced.
Why is ammonia relegated to the sidelines in most plant production systems?
In any case, ammonia (NH3) is relegated to the sidelines in most plant production systems because of its difficulty as a nitrogen fertilizer.
Why are there different kinds of plants?
The reason why there are different kinds of plants may also come from population dynamics. A population with both NH4+ uptakers and NO3- uptakers may prosper better than a plant community in which everybody prefers the same form of nitrogen. Please see the paper by S. Boudsocq and co-workers in American Naturalist 180: 60–69, 2012. DOI: 10.1086/665997.
What plants absorb nitrates?
There are plenty of aquatic plants you can put in your tank that absorb the most nitrate. The most popular one is the Hornwort, as it is really doing a fine job in reducing nitrates. But you can also try Duckweed, Moss Balls, Water Sprite , Anacharis Elodea, Frogbit, Water Lettuce, Vallisneria Spiralis, and Echinodorus Bleheri. Most of these plants grow faster, and plants that grow faster eats a lot of nitrates.
What are some good nitrate suckers?
If you want a great nitrate sucker, two other great floating plants are Salvinia Minima (Water Spangles) or Dwarf Water Lettuce. From what I have seen, these both are fairly available and very hardy. You could do something like red root floaters, but they are finicky and need almost 0 flow. Amazon Frogbit is good too, but I haven't had the same success as the two I recommended, it could work better for you. European frogbit is invasive, so no-no to that one. Best benefit to these is that once you get extra dwarf water lettuce, if your LFS doesn't have it, they will want it (in most cases). Salvinia minima isn't very sought after necessarily. Think of it as a deluxe duckweed. If you want it gone, its do-able, but it will still have the annoyance factor of sticking to you and being everywhere. Its more manageable.#N#As for nitrate suckers floating plants are going to be your best, unless you're running a high tech super advanced setup, and in that case you would probably already know what to get.#N#They are the best because they have access to open air, so basically infinite co2, and because they are closest to the lights, they get the most. This means that the limiting factor in growth is nutrients, which comes from the tank (nitrates).#N#I agree with the fact that at 10ppm you don't need it, but you wanted to know a good nitrate sucker, so here it is. Goodluck!
Did 2000 api test kits have nitrates?
It really was just salesmanship to sell products. In 2000 api test kits didn’t even have a nitrate test in it. People like to worry about stuff and that sells.
Do plants eat nitrates?
Plants do eat nitrates but not like you think. A well planted tank the plants will eat enough nitrate to starve the algae. The way to remove nitrate is water changes. A well balanced planted tank will have just a few fish. So few fish and many plants will give you a tank that doesn’t need water changes as often as our types of tanks.
Is duckweed a nitrate sink?
Duckweed is a complete nitrate sink, and provides a nice overhead environment to make the fish feel comfortable. It's also kind of tasty if you're into that kind of thing.