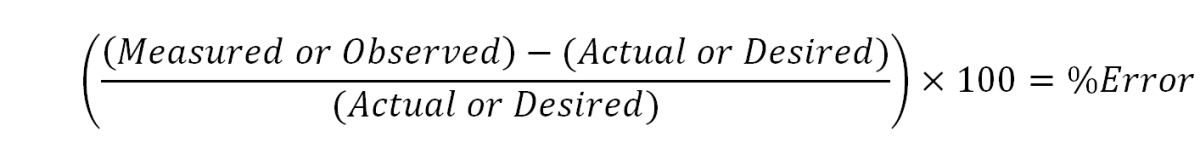Consider a percent error calculation where the measured value has 1 significant figure (XmL), and the actual/true value has 3 significant figures (0.XXXmL). If you only have one significant figure then you only have one significant figure.
Is there a special rule for percent error calculations?
Or is there a special rule for percent error calculations because it's a percent? There is no special rule for % conversion. So if you only have one significant figure then you'd have say 7 % or 7 × 10 1 %. You can't get 74 % conversion out of one significant figure.
Is the hundredth place in the percent a significant error?
If you write 0.00% it isn't entirely clear if that is two or three significant figures. But you have claimed that the hundredth place in the % is significant, which it is not. Remember that the minimum nonzero error possible was +/- 0.001 which yields an error of 0.1%.
What is error analysis and significant figures?
Error Analysis and Significant Figures. Errors using inadequate data are much less than those using no data at all. No measurement of a physical quantity can be entirely accurate. It is important to know, therefore, just how much the measured value is likely to deviate from the unknown, true, value of the quantity.
How many significant figures should I report on my report?
You should only report as many significant figures as are consistent with the estimated error. The quantity 0.428 m is said to have three significant figures, that is, three digits that make sense in terms of the measurement. Notice that this has nothing to do with the "number of decimal places".

Do you round off percent error?
example 15: If the number of significant figures of an experimental result is different from that of an accepted value, one of them should be rounded off so that both have the same number of significant figures in calculating percent errors.
How many significant figures should error have?
(1) The number of significant figures in the experimental uncertainty is limited to one or (when the experimental uncertainty is small, e.g., ± 0.15) to two significant figures. You should not use more than two significant digits when stating the experimental uncertainty.
What numbers do you use for percent error?
Percentage Error = ((Estimated Number – Actual Number)/ Actual number) x 100.
How many significant figures are in a percentage?
An analogous rule for percentages might be to use enough decimal places to ensure two significant digits for the range of values across groups, eg, if the range is 10% or more use whole numbers, if less than 1% use two decimal places, and otherwise one.
How do you report significant figures and errors?
Reporting it as 1.03 x 104 implies only three significant figures, meaning an uncertainty of ± 100. Reporting an uncertainty of 0.05 x 104 does not leave the impression that the uncertainty is ± 0.01 x 104, i.e., ± 100. A number reported as 10,300 ± 50 containing four significant figures.
What does a percent error of 3% mean?
For example, a 3% error means your estimated value is close to the real value, while a 30% error would mean your measured value was farther away from the accepted value.
What is the formula for calculating percentage error?
To calculate percentage error, you subtract the actual number from the estimated number to find the error. Then, you divide the error in absolute value by the actual number in absolute value. This gives you the error in a decimal format. From there, you can multiply by 100% to find the percentage error.
How do you interpret percent error?
Percent errors tells you how big your errors are when you measure something in an experiment. Smaller values mean that you are close to the accepted or real value. For example, a 1% error means that you got very close to the accepted value, while 45% means that you were quite a long way off from the true value.
How do you represent errors?
To represent random error, we commonly use what we call an error bar, consisting of a vertical line that extends from the mean value in proportion to the magnitude of the error. The most common type of error bar that you will encounter includes a "cap" that clearly indicates the end of the bar in each direction.
What are significant errors?
Significant Error means an error, defect or omission that causes the Wellnomics Software to be unusable in large part by Users.
How many decimal places should uncertainty have?
Uncertainties are almost always quoted to one significant digit (example: ±0.05 s). If the uncertainty starts with a one, some scientists quote the uncertainty to two significant digits (example: ±0.0012 kg). Always round the experimental measurement or result to the same decimal place as the uncertainty.
How is an error calculated?
Percent Error Calculation Steps Subtract the theoretical value from the experimental value if you are keeping negative signs. This value is your "error." Divide the error by the exact or ideal value (not your experimental or measured value).
Why is the percent error not distinguishable?
So, due to the limitations of the method used to determine the density, the experimental value and accepted value are not distinguishable from each other and that a percent error cannot be determined unless the experiment is redone in a way which increases precision.
What is the minimum nonzero error?
Remember that the minimum nonzero error possible was +/- 0.001 which yields an error of 0.1%.
What is the sig fig of 0.997?
Here is the more important part and the rational behind the sig fig rules... 0.997 g/mL implies a precision of +/- 0.001g/ml. The accepted value of 0.997171 g/mL falls within this range. So, due to the limitations of the method used to determine the density, the experimental value and accepted value are not distinguishable from each other and that a percent error cannot be determined unless the experiment is redone in a way which increases precision.
Can a sig fig slide with percent error?
FYI, my experience is the most Gen Chem teachers let sig fig errors slide with percent error since this concept can be difficult to understand.
Is 0.00% a significant figure?
You could write that the error was 0% but that would indicate only one part in 100 precision. If you write 0.00% it isn't entirely clear if that is two or three significant figures. But you have claimed that the hundredth place in the % is significant, which it is not.
Is the percent error zero?
I believe that you are correct in saying that the percent error is zero in this case, which is what I tell my gen chem students who do not know how to statistically evaluate uncertainty. As the previous answer-er pointed out, "when subtracting, Addition and subtraction round to the the last common significant decimal place of all the measurements." The last common sig DP between 0.997 and 0.99717 is the third decimal place, so the result of your subtraction, when rounded to the correct number of sig figs is zero.
Confused about teacher comment
Hello! I had an assignment where one of the questions was what type of compound NaOH is. I said it’s an ionic compound. Just got it back and teacher took off a mark and said that it’s technically a polyatomic compound.
Chirality of amines
Is it possible for a secondary or tertiary amine to be considered as a chiral center? Ot does only have 3 substituends, but can the full orbital be considered as one too, making it chiral? Or is it able to freely switch places with another substituend making it achiral?
Balancing Chemistry Equations
Hey! I'm having some trouble with this topic. I was away on vacation when we were taught this but I haven't had the time to fully learn it yet and my finals are tomorrow. Can someone help explain how to balance equations?
why is Manganese Dioxide (Mno2) insoluble
Why is Manganese Dioxide (MnO2) not soluble in water or covalent substances such as cloroform?
Calculating change in entropy
So let’s say you’re cooling and expanding a gas simultaneously. How would I know if the change in entropy is positive or negative?
What is the purpose of percent error?
The purpose of a percent error calculation is to gauge how close a measured value is to a true value.
When keeping the sign for error, what is the calculation?
When keeping the sign for error, the calculation is the experimental or measured value minus the known or theoretical value, divided by the theoretical value and multiplied by 100%.
How to find the decimal number of an error?
Divide the error by the exact or ideal value (not your experimental or measured value). This will yield a decimal number.
Is percent error always positive?
For many applications, percent error is always expressed as a positive value. The absolute value of the error is divided by an accepted value and given as a percent.
How many decimal places are there in a percentage?
If your denominator is $1000$then one decimal place in the percentage is the exact answer but reporting only the integer part will be easier to read. If your denominator is $1,000,000$then four decimal places will be exact but zero or one or two may best convey what you are trying to say. If your denominator is $10$just report the count.
What level of precision should you choose?
In general, you should choose the level of precision that roughly matches the precision of the input and the kind of information you want to convey. In your case the numerator and denominator are counts, which you presumably know exactly.
What to do if your denominator is 10?
If your denominator is 10 just report the count. For more advice you could edit the question to provide more context. Percentages do not differ from "ordinary" values. In every case, you are deemed to know how many digits are significant (i.e. exact) and how many are useful for the application at hand.