
What is the reactivity order of alcohol with HBr?
When treated with HBr or HCl alcohols typically undergo a nucleophilic substitution reaction to generate an alkyl halide and water. Alcohol relative reactivity order : 3o > 2o > 1o > methyl. Methanol and primary alcohols will proceed via an SN2 mechanism since these have highly unfavourable carbocations. Click to see full answer.
What is the reaction between HBr and HCl?
When treated with HBr or HCl alcohols typically undergo a nucleophilic substitution reaction to generate an alkyl halide and water. Alcohol relative reactivity order : 3o > 2o > 1o > methyl. Methanol and primary alcohols will proceed via an SN2 mechanism since these have highly unfavourable carbocations.
What happens when you add HBr to an alkylene?
HBr adds to alkenes to create alkyl halides. A good way to think of the reaction is that the pi bond of the alkene acts as a weak nucleophile and reacts with the electrophilic proton of HBr.
How do tertiary alcohols react with hydrogen halides?
Typically, tertiary alco- hols react with hydrogen halides rapidly at room temperature, whereas the reactions of pri- mary alcohols require heating for several hours. The reactions of primary alcohols with HBr and HI are satisfactory, but their reactions with HCl are very slow.
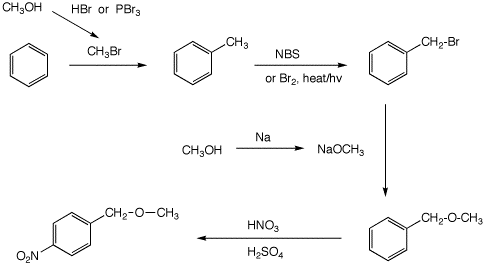
Can ethanol react with HBr?
When treated with HBr or HCl alcohol typically undergo a nucleophilic substitution reaction to generate an alkyl halide and water.
Does HCl react with alcohol?
Tertiary alcohols react reasonably rapidly with concentrated hydrochloric acid, but for primary or secondary alcohols the reaction rates are too slow for the reaction to be of much importance. A tertiary alcohol reacts if it is shaken with with concentrated hydrochloric acid at room temperature.
How do alcohols react with bromine?
alcohol (by weight) and bromine in a molar ratio of two to one react at 25°, ethyl acetate and hy- drobromic acid are the sole reaction products. respect to free bromine. The tribromide ion formed during the reaction does not react with alcohol.
What do alcohols react with?
Alcohols may be oxidized to give ketones, aldehydes, and carboxylic acids. These functional groups are useful for further reactions; for example, ketones and aldehydes can be used in subsequent Grignard reactions, and carboxylic acids can be used for esterification.
Why tertiary alcohol react with HBr faster than secondary alcohol?
Tertiary alcohols undergo substitution reactions with hydrogen halides faster than secondary alcohols do because tertiary carbocations are more stable and, therefore, are formed more rapidly than secondary carbocations.
What is the order of reactivity of HCl HBr and HI with alcohol?
The order of reactivity of alcohols is 3° > 2° > 1° methyl. The order of reactivity of the hydrogen halides is HI > HBr > HCl (HF is generally unreactive).
Does alcohol react with bromine water?
In aliphatic compounds of alcohol has no reaction with bromine water. So here ethanol does not react with bromine water.
What happens when you add bromine water to an alcohol?
Bromine water is an orange solution of bromine. It becomes colourless when it is shaken with an alkene. Alkenes can decolourise bromine water, but alkanes cannot.
How do alkenes react with bromine?
Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The double bond breaks, and a bromine atom becomes attached to each carbon. The bromine loses its original red-brown color to give a colorless liquid.
What makes alcohol more reactive?
The tertiary alcohol is more reactive than other alcohols because of the presence of the increased number of alkyl groups. These alkyl group increases the +I effect in the alcohol.
Do all alcohols react with sodium?
Alcohols react with sodium to form a salt (sodium alkoxide) and hydrogen gas. The reaction is similar but much slower than the reaction of water and sodium. This is because of the similarities in the structure of the water molecule and the alkyl (O—H) group in alcohols.
Why are alcohols reactive?
Alcohol Reactions. The functional group of the alcohols is the hydroxyl group, –OH. Unlike the alkyl halides, this group has two reactive covalent bonds, the C–O bond and the O–H bond. The electronegativity of oxygen is substantially greater than that of carbon and hydrogen.
What is the hydroxyl group of alcohol?
The hydroxyl group of an alcohol is replaced by halogen on reaction with concentrated halogen acid ( H X, where X is halogen), to get alkyl halides. Usually we don't need boiling the reactants. But, for the preparation of alkyl bromide requires constant boiling of alcohol with H B r ( 48 % ).
What are halogenations with these four acids?
Of course, halogenations with these four acids are reactions of equilibria, and these may be driven forward by increasing the concentration of the reactants, for example. And I do recall two-step protocols where the alcohol is activated by sulfuric acid before adding hydrobromic acid.
What is the reaction that occurs when alcohol is mixed with deuterium oxide?
Another such substitution reaction is the isotopic exchange that occurs on mixing an alcohol with deuterium oxide (heavy water). This exchange, which is catalyzed by acid or base, is very fast under normal conditions, since it is difficult to avoid traces of such catalysts in most experimental systems.
Which is the most reactive site in an alcohol molecule?
The most reactive site in an alcohol molecule is the hydroxyl group, despite the fact that the O–H bond strength is significantly greater than that of the C–C, C–H and C–O bonds, demonstrating again the difference between thermodynamic and chemical stability.
What is the functional group of alcohols?
The functional group of the alcohols is the hydroxyl group, –OH. Unlike the alkyl halides, this group has two reactive covalent bonds, the C–O bond and the O–H bond. The electronegativity of oxygen is substantially greater than that of carbon and hydrogen.
Why is the hydrogen atom on the hydroxyl group easily replaced by other substituents?
Because of its enhanced acidity, the hydrogen atom on the hydroxyl group is rather easily replaced by other substituents. A simple example is the facile reaction of simple alcohols with sodium (and sodium hydride), as described in the first equation below.
What is the mechanism by which many substitution reactions of this kind take place?
The mechanism by which many substitution reactions of this kind take place is straightforward. The oxygen atom of an alcohol is nucleophilic and is therefore prone to attack by electrophiles. The resulting "onium" intermediate then loses a proton to a base, giving the substitution product.
What suffix is used for hydroxyl functional group?
Many functional groups have a characteristic suffix designator, and only one such suffix (other than "ene" and "yne") may be used in a name. When the hydroxyl functional group is present together with a function of higher nomenclature priority, it must be cited and located by the prefix hydroxy and an appropriate number.
What does the number in parentheses mean in 2o alcohol?
The numbers in parentheses next to the mineral acid formulas represent the weight percentage of a concentrated aqueous solution, the form in which these acids are normally used.
What is the product of oxidation of primary alcohols?
Oxidation of Primary Alcohols Primary alcohols are easily oxidized just like secondary alcohols, and the INITIAL product of oxidation is an aldehyde.
What reagent is used to oxidize alcohol?
Oxidation of Alcohols Primary and secondary alcohols are easily oxidized by a variety of reagents. Secondary Alcohols The most common reagent used for oxidation of secondary alcohols to ketones is chromic acid, H. 2.
What are the limitations of H-X?
Limitations of use of H-X 1) Only works for H-Cl and H-Br 2) Low chemical yields for primary and secondary alcohols 3) Often observe competing elimination 4) Carbocations can lead to rearranged products . Phosphorous Halides Phosphorous halides can convert alcohols to alkyl halides. E.g. 3 R-OH + PCl.
How does alcohol dehydration occur?
Alcohol dehydration usually occurs via the E1 mechanism. Ch11 Reacns of Alcohols (landscape).docx Page 16 . Alcohol dehydration usually occurs via the E1 mechanism. The first step is the exothermic protonation of the hydroxyl, followed by the slow, endothermic, rate determining ionization to generate the cation.
Is alcohol an organic compound?
Alcohols are versatile organic compounds since they undergo a wide variety of transformations – the majority of which are either oxidationor reductiontype reactions. Normally: Oxidation is a loss of electrons; Reduction is a gain of electrons. But in organicterms: .
Is alcohol in equilibrium?
In acidic media, the alcohol is in equilibrium with its protonated form. The –OH is a poorleaving group, but –OH. 2 +is an excellentleaving group, and once the -OH is protonated, the molecule may take part in a variety of substitution and/or elimination reactions.
Can aldehyde be oxidized?
However, the aldehydecan also be easily oxidized to an acid, and this ‘over-oxidation’ is a practical problem. E.g. . A common reagent that selectively oxidizes a primary alcohol to an aldehyde (and no further) is pyridinium chlorochromate, PCC.
