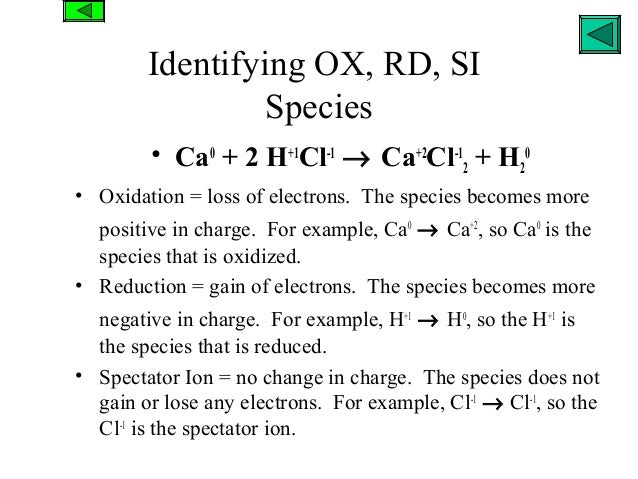
The following thought pattern can be helpful:
- An oxidizing agent oxidizes something else.
- Oxidation is loss of electrons (OIL RIG).
- Therefore, an oxidizing agent takes electrons from that other substance.
- Therefore, an oxidizing agent must gain electrons.
Does oxidation lose or gain electrons?
· An oxidizing agent, or oxidant, gains electrons and is reduced in a chemical reaction. Also known as the electron acceptor, the oxidizing agent is normally in one of its higher possible oxidation states because it will gain electrons and be reduced.
What happens when a molecule is oxidized?
· An oxidizing agent (often referred to as an oxidizer or an oxidant) is a chemical species that tends to oxidize other substances, i.e. cause an increase in the oxidation state of the substance by making it lose electrons. Common examples of oxidizing agents include halogens (such as chlorine and fluorine), oxygen, and hydrogen peroxide (H 2 O 2).
How do oxidizing agents undergo reduction?
· Oxidation occurs when a molecule loses an electron or increases its oxidation state. When a molecule is oxidized, it loses energy. In contrast, when a molecule is reduced, it gains one or more electrons. As you might have guessed, the molecule gains energy in the process. Confused? Think about it like this.
What happens when electrons are lost or gained in a reaction?
· Oxidizing agents accept electrons (gain electrons), while reducing agents give electrons (lose electrons).

Is oxidizing losing or gaining?
Oxidation occurs when a molecule loses an electron or increases its oxidation state. When a molecule is oxidized, it loses energy. In contrast, when a molecule is reduced, it gains one or more electrons. As you might have guessed, the molecule gains energy in the process.
Does oxidizing lose electrons?
Oxidation is a combination of elements with oxygen. It's also a reaction of losing electrons and gaining positive charge. The atoms that lost electrons are said to be oxidized. Atoms can be oxidized by nonmetals.
Why do oxidising agents gain electrons?
The oxidizing agent is a substance that causes oxidation by accepting electrons; therefore, its oxidation state decreases. The reducing agent is a substance that causes reduction by losing electrons; therefore its oxidation state increases.
Does the oxidation gain electrons?
Oxidation is the loss of electrons, gain of oxygen or loss of hydrogen. Reduction is the gain of electrons, loss of oxygen or gain of hydrogen.
What is the oxidizing agent?
An oxidizing agent is a compound or element that is present in a redox (oxidation-reduction) reaction which receives electrons originating from a different species. The oxidant is a chemical compound which easily transfers atoms of oxygen or another substance in order to gain an electron.
What is the difference between oxidising agent and reducing agent?
Figure 1: A reducing agent reduces other substances and loses electrons; therefore, its oxidation state increases. An oxidizing agent oxidizes other substances and gains electrons; therefore, its oxidation state decreases.
What is meant by oxidising agent and reducing agent?
: An oxidizing agent is an element that gains electrons. Since the oxidizing agent means to gain electrons; it is said to have been reduced. The element which undergoes reduction (gets reduced) is called an oxidizing agent.
How do you identify oxidizing agent and reducing agent?
6:3212:21How To Find The Oxidizing and Reducing Agent - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo remember the substance that is oxidized is the reduce an agent and the substance that is reducedMoreSo remember the substance that is oxidized is the reduce an agent and the substance that is reduced is the oxidizing agent now let's work on another example hydrogen gas reacts with oxygen gas to
What is reducing agent in chemistry?
A reducing agent in chemistry is a compound that easily loses electrons, thus increasing its oxidation state and being oxidized.
What is a oxidizing agent in chemistry?
An oxidizing agent in chemistry is a compound that easily gains electrons, thus decreasing its oxidation state and being reduced.
How do you identify oxidizing and reducing agents?
Oxidizing and reducing agents are identified by looking at the starting and ending oxidation charges of compounds in a reaction. Oxidizing agents d...
What is an oxidizing agent?
An oxidizing agent (often referred to as an oxidizer or an oxidant) is a chemical species that tends to oxidize other substances, i.e. cause an increase in the oxidation state of the substance by making it lose electrons. Common examples of oxidizing agents include halogens (such as chlorine and fluorine), oxygen, and hydrogen peroxide (H 2 O 2 ).
What are the processes that require oxidizing agents?
Combustion of fuel involves the use of an oxidizing agent. Storage of energy in batteries. Vulcanization of rubber (increasing the strength and the elasticity of rubber). Oxidizing agents are also vital to many biological processes such as metabolism and photosynthesis.
What is the formula for hydrogen peroxide?
Hydrogen Peroxide. Hydrogen peroxide is the chemical compound having formula H 2 O 2. It appears to the human eye as a colourless liquid which has a greater viscosity than water. Hydrogen peroxide is the simplest compound having a peroxide functional group with an oxygen-oxygen single bond.
What is the atomic number of oxygen?
Oxygen. Oxygen is the element corresponding to the atomic number 8 and is denoted by the symbol ‘O’. It belongs to the chalcogen group of the periodic table and is a highly reactive non- metal with good oxidizing properties.
Which group of elements have the highest electronegativities?
Halogens. The group 17 elements of the periodic table are collectively referred to as Halogens. They are said to have a strong ability to gain electrons, attributed to their high electronegativities when compared to elements from other groups.
What are some examples of oxidizers?
Acidic examples of good oxidizers include nitric acid, perchloric acid, and sulphuric acid. The electronegativity of the molecules increases with the increase in the oxidation states of the atoms, increasing their ability to oxidize other substances.
Which compounds are good oxidizing agents?
Ionic examples include the permanganate ion, the chromate ion, and the dichromate ion.
When a molecule is oxidized, does it gain or lose energy?
Updated September 06, 2018. If a molecule is oxidized, does it gain or lose energy? Oxidation occurs when a molecule loses an electron or increases its oxidation state. When a molecule is oxidized, it loses energy. In contrast, when a molecule is reduced, it gains one or more electrons.
Do electrons have more energy?
Electrons orbit the atomic nucleus, giving it electrical and kinetic energy. If you have more electrons, you have more energy . Keep in mind, however, energy input may be required (activation energy) to get a molecule to change its oxidation state. Cite this Article.
Is rusting iron an oxidation reaction?
Rusting of iron is an example of an oxidation reaction. Watcharapong Thawornwichian / EyeEm / Getty Images
Oxidizing Agent
An oxidizing agent is a compound that oxidizes other compounds. Oxidizing agents can also be called oxidants or oxidizers. The process of oxidation occurs when the oxidizing agent accepts electrons from another compound, called the reducing agent.
What is a Reducing Agent?
Now that we've looked at oxidizing agents, what is a reducing agent? A reducing agent is the opposite (or the compound causing the reaction to go the opposite direction) of the oxidizing agent, and it reduces other compounds. It does this by giving up electrons to the oxidizing agent, and the reducing agent itself becomes oxidized in the process.
Oxidizing Agent vs Reducing Agent
Oxidizing and reducing agents always occur in the same reactions, because if one compound releases an electron then there must be another compound to receive it. The main differences between oxidizing agents versus reducing agents include the following:
Identifying Oxidizing and Reducing Agents
In order identify oxidizing and reducing agents, we need to look at specific examples of chemical reactions. An oxidizing agent will:
What is the difference between oxidation and reduction?
A species that undergoes oxidation is conceived to lose electrons. On the other hand, a species that undergoes reduction is conceived to gain electrons.
Is carbon dioxide a zerovalent reactant?
Both reactants, are zerovalent, and are presumed to have neither accepted nor donated electrons. The carbon dioxide is the product of the redox reaction where the reducing reactant, carbon, has donated 4 electrons to the oxidizing oxygen, which clearly has been reduced in the product.
Does every oxidation have a reduction?
But for every electron loss, for every oxidation, there is a corresponding electron gain, a corresponding reduction. And here, clearly, the oxygen has been reduced. Capisce?
What happens to the reducing agent when it oxidizes?
In a corrosive process, the anode oxidizes and the cathode reduces. Stated differently, the reducing agent loses electrons and corrodes while the oxidizing agent gains electrodes. Advertisement.
What is the atom where the reducing agent sends its electron or electrons called?
The atom where the reducing agent sends its electron or electrons is called the oxidant. The reducing agent causes the oxidant to become reduced. Corrosion occurs because of reducing agents and oxidizing agents. The oxidation of the reducing agent causes it to become corroded. In a corrosive process, the anode oxidizes and the cathode reduces.
Which elements are reducing agents?
Examples of reducing agents include zinc, lithium, iron and oxalic acid. Proper knowledge and use of reducing agents can help prevent oxidation of some materials.
What does reducing mean in chemistry?
What Does Reducing Agent Mean? A reducing agent is a substance with atoms that lose, or gives up, electrons in a chemical reaction. When a reducing agent gives up an electron or electrons, it is considered to be oxidized.
What can help prevent oxidation?
Proper knowledge and use of reducing agents can help prevent oxidation of some materials. Take galvanized steel for example. The zinc coating on the steel helps prevent corrosion, even, to an extent, if the coating is damaged.
