What diseases are caused by anemia?
- Heart problems. Iron deficiency anemia may lead to a rapid or irregular heartbeat. ...
- Problems during pregnancy. In pregnant women, severe iron deficiency anemia has been linked to premature births and low birth weight babies. ...
- Growth problems. In infants and children, severe iron deficiency can lead to anemia as well as delayed growth and development. ...
Why does chronic blood loss cause anemia?
What causes anemia in CKD?
- blood loss, particularly if you are treated with dialysis for kidney failure
- infection
- inflammation
- malnutrition, a condition that occurs when the body doesn’t get enough nutrients
What is the treatment for anemia of inflammation?
- Treat the underlying disease
- Treat anemia specifically only if severe or limits activities of daily living
- Erythrocyte transfusion for acute symptoms
- Erythropoiesis-stimulating agents (ESAs) with or without IV iron (off label treatment)
How do you treat anemia of chronic disease?
chronic kidney disease (CKD) is a key factor fuelling demand for anemia treatment. Anemia treatment market is also receiving a strong impetus from the rising popularity of combination therapy. Increasing use of vitamin and iron supplements, antibiotics ...

Can low iron cause inflammation?
High intracellular iron also downregulates transferrin production, lowering TIBC. Iron release is so restricted that the decrease in serum iron still lowers TSAT despite low TIBC. Iron restriction eventually leads to the anemia of inflammation.
Can anemia cause high inflammation markers?
Other features of anemia of inflammation include inappropriately low levels of erythropoietin, and elevated measures of inflammatory markers, such as C-reactive protein [2].
What problems do anemia cause?
Anemia can lead to a rapid or irregular heartbeat (arrhythmia). When you're anemic your heart pumps more blood to make up for the lack of oxygen in the blood. This can lead to an enlarged heart or heart failure.
Can anemia cause body swelling?
Background: Patients with chronic severe anaemia often retain salt and water. Fluid retention in these patients is not caused by heart failure and the exact mechanisms remain unclear.
What is the main cause of inflammation in the body?
Causes of an inflammation Pathogens (germs) like bacteria, viruses or fungi. External injuries like scrapes or damage through foreign objects (for example a thorn in your finger) Effects of chemicals or radiation.
Can bloodwork tell if you have inflammation?
Blood tests known as 'inflammatory markers' can detect inflammation in the body, caused by many diseases including infections, auto-immune conditions and cancers. The tests don't identify what's causing the inflammation: it might be as simple as a viral infection, or as serious as cancer.
What 3 conditions would cause anemia?
Possible causes of anemia include:Iron deficiency.Vitamin B12 deficiency.Folate deficiency.Certain medicines.Destruction of red blood cells earlier than normal (which may be caused by immune system problems)Long-term (chronic) diseases such as chronic kidney disease, cancer, ulcerative colitis, or rheumatoid arthritis.More items...
What are the 3 main causes of anemia?
Your body needs iron to make hemoglobin. Hemoglobin is an iron-rich protein that gives the red color to blood. It carries oxygen from the lungs to the rest of the body. Anemia has three main causes: blood loss, lack of red blood cell production, and high rates of red blood cell destruction.
What does severe anemia feel like?
Anemia occurs when there aren't enough healthy red blood cells to carry oxygen to your body's organs. As a result, it's common to feel cold and symptoms of tiredness or weakness. There are many different types of anemia, but the most common type is iron-deficiency anemia.
How is chronic inflammation anemia treated?
How do you correct anemia of chronic disease?Blood transfusion: Providers may use blood transfusions as a short-term therapy to help people who have severe anemia. ... Synthetic EPO therapy: This treatment boosts your EPO levels. ... Iron supplements: Providers may combine EPO therapy and iron supplement therapy.
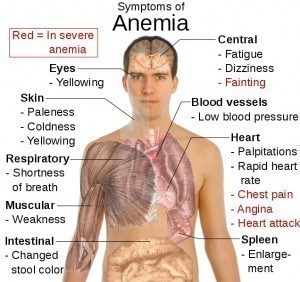
What is the most common symptom and complication of anemia?
With all forms of anemia, tiredness or fatigue is the most common symptom because of low red blood cell count. Shortness of breath, dizziness, headache, coldness in your hands and feet, pale or yellowish skin, and chest pain are other signs.
Can low iron cause joint and muscle pain?
Fatigue and neurocognitive symptoms often raise a suspicion of depression. Furthermore, headache and muscle and joint pain associated with iron deficiency are repeatedly considered migraine and fibromyalgia syndrome, respectively 3, 19.
Does anemia cause high CRP?
Data indicate that CRP levels correlate with anemia parameters, higher levels being associated with an increased comorbidity burden, lower hemoglobin (Hb) levels, and higher Epoetin alfa dose requirements.
Can low iron cause elevated CRP?
Iron deficiency at any yearly time point was associated with higher increases in hs-CRP (mean difference in change: 1.62 mg/L, 95%CI 0.98–2.26, P < . 001) and IL-6 levels (mean difference in change: 1.33 ng/L, 95%CI 0.87–1.79, P < .
Can anemia cause high CRP and ESR?
Any condition that elevates fibrinogen (e.g., pregnancy, diabetes mellitus, end-stage renal failure, heart disease, collagen vascular diseases, malignancy) may also elevate the ESR. Anemia and macrocytosis increase the ESR.
Can low hemoglobin cause high CRP?
There was an inverse relationship between Hb and CRP levels in both sexes (men: r = ‒0.88; P = . 0006; women r = ‒0.65; P = . 012). Conclusions: Anemia was prevalent in the HS population, and Hb levels inversely correlated with CRP.
What is the pathogenesis of AI?
The pathogenesis of AI is mediated by inflammatory cytokines and hepcidin, acting together to suppress erythropoiesis and shorten erythrocyte survival in blood. The effects of cytokines are denoted in light green, hepcidin effects are depicted in orange, and combined effects in red.
What is the pathophysiology of AI?
Despite more than fifty years of investigation, our understanding of the pathophysiology of AI is incomplete. Already the earliest studies of AI indicated that the disorder is a consequence of a mild decrease in erythrocyte survival combined with impaired production of erythrocytes1;20. The increased destruction of erythrocytes is predominantly attributable to macrophage activation by inflammatory cytokines but other hemolytic mechanisms may contribute in specific inflammatory diseases. The suppression of erythrocyte production has two major components, iron restriction and direct cytokine effects on erythropoietic progenitors. These effects combine to limit the erythropoietic response to erythropoietin which becomes insufficient to compensate for the increased destruction of erythrocytes. In some situations, the production of erythropoietin may also be decreased, perhaps due to cytokine effects on the renal cells that produce the hormone. In severe inflammation, or when the primary pathology involves the kidneys, decreased renal excretion of hepcidin contributes to hepcidin accumulation and iron restriction21. The complex pathogenesis of AI is summarized in Figure 1and discussed further.
What is the gold standard for AI?
The traditional gold standard for the diagnosis of AI was anemia with hypoferremia or with low transferrin saturation , despite the presence of Prussian-blue-stainable iron in bone marrow macrophages. The main confounding diagnostic entity that also presents with anemia and hypoferremia is iron deficiency anemia where there is no stainable iron in the marrow macrophages. This gold standard has been challenged not only because of the invasive nature of the marrow sampling procedure but also because of findings that bone marrow iron readings are qualitative and not always consistent between evaluators and in multiple specimens6;7and that iron therapy may cause marrow iron deposition in a poorly bioavailable form which cannot be used by iron-deficient patients8. The marrow iron stain has largely been replaced by serum ferritin determinations. Low serum ferritin (less than 15 ng/ml for general population with some labs using age and gender-specific norms) is highly specific for iron deficiency9(genetic deficiency of L-ferritin is an extremely rare exception10) and effectively rules out AI. AI is diagnosed when anemia and hypoferremia are accompanied by serum ferritin that is not low. Serum ferritin is increased by inflammation, in part reflecting direct inflammatory regulation of ferroportin synthesis11;12and in part because serum ferritin originates in macrophages where its synthesis is increased by iron sequestration13that takes place during inflammation. Iron deficiency is presumed to coexist with AI when ferritin is insufficiently elevated for the intensity of inflammation. Serum ferritin is also increased by tissue injury, especially to the liver.
What is anemia of chronic disease?
Anemia of inflammation (AI, also called anemia of chronic disease) is a common, typically normocytic normochromic anemia that is caused by an underlying inflammatory disease . It is diagnosed when serum iron concentrations are low despite adequate iron stores, as evidenced by serum ferritin that is not low. In the setting of inflammation, AI may be difficult to differentiate from iron deficiency anemia, and the two conditions may coexist. In AI, erythropoiesis is iron-restricted by hepcidin-mediated hypoferremia and erythrocyte production is suppressed by cytokines acting on erythroid progenitors. Decreased erythropoiesis is unable to compensate for shortened erythrocyte lifespan caused by enhanced erythrophagocytosis by cytokine-activated macrophages. Treatment should focus on the underlying disease. If this is not feasible and the anemia limits the quality of life or the performance of daily activities, a combination of erythropoiesis-stimulating agents and intravenous iron may be effective but should be attempted only after careful consideration of risk and benefit. Recent advances in molecular understanding of AI are stimulating the development of new pathophysiologically targeted experimental therapies.
What cytokines suppress erythropoiesis?
Inflammatory cytokines , including TNFα, IL-1 and interferon-γ, have been reported to suppress erythropoiesis in vitro 37-41as well as in mouse models24;42. Detailed understanding of the mechanisms involved has been hindered by the complexity of cytokine effects and the ability of each cytokine to regulate the production of many other cytokines41. Nevertheless, several new and promising concepts about the effects of cytokines on erythropoiesis have recently emerged. Libregts at al24developed a mouse model where overproduction of interferon-γ leads to the development of a mild-to-moderate normocytic normochromic anemia. The model manifests a 50% decrease in erythrocyte survival attributable to interferon-γ-mediated activation of macrophages in the splenic red pulp. The model also shows suppression of erythrocyte production affecting the erythroblast stages and the earliest erythroid-committed precursor BFU-e (burst-forming unit-erythrocyte) but not proerythroblasts and CFU-e (colony-forming unit-erythrocyte). Importantly, myeloid CFU-G/M (colony-forming unit-granulocyte/macrophage) colonies were increased. Microarray analysis of erythroblasts indicated that interferon-γ promotes the transcription of PU.1 and its target genes in an IRF-1-dependent manner but does not affect GATA-1 or its targets. PU.1 and GATA-1 antagonize each other's activity so the increase in PU.1 would be expected to promote myelopoiesis at the expense of erythropoiesis. During infections with viruses or intracellular pathogens known to induce interferon-γ, this mechanism may assure sufficient production of monocytes and macrophages, at the expense of temporary impairment of erythropoiesis. Whether other inflammatory cytokines utilize a similar or different mechanism remains to be determined.
What is the lagging indicator of anemia?
The defining biochemical features of AI include low serum iron despite adequate systemic iron stores. The concentration of serum transferrin is also decreased during chronic inflammation but this is a lagging indicator because of the long half-life of transferrin (about 8 days) compared to iron (about 1.5 hours)2. The erythrocytes are usually of normal size and have normal hemoglobin content but are reduced in number (normocytic, normochromic anemia). In some cases, particularly if the inflammatory disease is longstanding, the red cells can be mildly decreased in size and hemoglobin content.
Does hepcidin cause anemia?
Increased hepcidin causes an iron-restricted anemia even in the absence of inflammation
What are the mechanisms of AI?
Systemic inflammation results in immune cell activation and formation of numerous cytokines. Interleukin (IL-6) and IL-1β, as well as lipopolysaccharide (LPS), are potent inducers of the master regulator of iron homeostasis, hepcidin, in the liver, whereas expression of the iron-transport protein transferrin is reduced. Hepcidin causes iron retention in macrophages by degrading the only known cellular iron exporter ferroportin (FP1); by the same mechanism, it blocks dietary iron absorption in the duodenum. Multiple cytokines (eg, interleukin-1β [IL-1β], IL-6, IL-10, and interferon-γ [IFN-γ]) promote iron uptake into macrophages, increase radical-mediated damage to erythrocytes and their ingestion by macrophages, and cause efficient iron storage by stimulating ferritin production and blocking iron export by transcriptional inhibition of FP1 expression. This results in the typical changes of AI (ie, hypoferremia and hyperferritinemia). In addition, IL-1 and TNF inhibit the formation of the red cell hormone erythropoietin (Epo) by kidney epithelial cells. Epo stimulates erythroid progenitor cell proliferation and differentiation, but the expression of its erythroid receptor (EpoR) and EpoR-mediated signaling are inhibited by several cytokines. Moreover, cytokines can directly damage erythroid progenitors or inhibit heme biosynthesis via radical formation or induction of apoptotic processes. Importantly, because of iron restriction in macrophages, the availability of this metal for erythroid progenitors is reduced. Erythroid progenitors acquire iron mainly via transferrin-iron/transferrin receptor (Tf/TfR)-mediated endocytosis. Erythroid iron deficiency limits heme and hemoglobin (Hb) biosynthesis, as well as reduces EpoR expression and signaling via blunted expression of Scribble (Scb). In addition, the reduced Epo/EpoR signaling activity impairs the induction of erythroferrone (Erfe), which normally inhibits hepcidin production.
What are the pathogenic factors of AI?
The second pathogenic factor in AI is iron- and hepcidin-independent impairment of erythropoiesis. It is caused, in part, by reduced production and/or reduced biological activity of the hormone Epo in the inflammatory setting. 34 Observational studies have indicated lower Epo levels than expected for the degree of anemia in most AI subjects. 1 These observations may be due, in part, to the inhibitory effects of cytokines, such as IL-1 and TNF, on hypoxia-mediated stimulation of Epo by interfering with mediated GATA-2 or HNF4 transcription or by causing radical-mediated damage of Epo-producing kidney epithelial cells. 35, 36 Epo exerts its biological effects after binding to its homodimeric erythroid receptor via JAK/STAT-mediated signaling cascades. 36 Although, the number of Epo receptors (EpoRs) did not appear to be altered in subjects with inflammatory anemia, the efficacy of Epo-mediated signaling is reduced and inversely linked to the circulating levels of IL-1 and IL-6, 37 indicating the inflammation-driven hyporesponsiveness of EpoRs. However, recent evidence suggests that erythroid iron deficiency, because it also occurs in AI, results in downregulation of EpoR, which could be traced back to iron-mediated regulation of the EpoR control element Scribble. 31 Specifically, iron deficiency impairs transferrin receptor-2–mediated iron sensing in erythroid cells, 38 resulting in downregulation of Scribble and reduced EpoR expression.
What cytokines affect iron absorption?
In addition, various cytokines directly impact on duodenal or macrophage iron homeostasis. Tumor necrosis factor (TNF) reduces duodenal iron absorption by a poorly characterized, but hepcidin-independent, mechanism. 20 The cytokines IL-1, IL-6, IL-10, or TNF-α promote iron acquisition into macrophages via transferrin receptor–mediated endocytosis, via divalent metal transporter 1, or possibly also via increased iron acquisition by lactoferrin and lipocalin-2. 21 However, the major source for iron for macrophages is senescent erythrocytes. Cytokines, inflammation-derived radicals, and complement factors damage erythrocytes and promote erythrophagocytosis via stimulation of receptors recognizing senescent red blood cells, such as T-cell immunoglobulin domain 4 or possibly CD44. 22, 23 Recent evidence suggests that, during periods of increased erythrocyte destruction, erythrophagocytosis and iron recycling are primarily carried out by hepatic macrophages differentiating in the liver from circulating monocytes, rather than by resident splenic macrophages. 24 Once iron is acquired by macrophages, it is mainly stored within ferritin, the major iron storage protein whose expression is massively induced by macrophage iron, heme, and cytokines. 5, 25 Although circulating and, to a lesser extent, macrophage-derived hepcidin are the main regulators of iron export by these cells, 15, 16, 18, 19, 26, 27 bacterial lipopolysaccharides and interferon-γ (IFN-γ) block the transcription of ferroportin, thereby reducing cellular iron export. 28, 29 All of these events lead to iron-restricted erythropoiesis and the characteristic changes in systemic iron homeostasis observed in AI: hypoferremia and hyperferritinemia. These effects are partially counteracted by the stimulation of synthesis of ferroportin in macrophages by retained iron and heme, 30 perhaps explaining why AI rarely reaches the severity seen in pure iron-deficiency anemia.
What is the therapeutic principle of prolyl hydroxylase inhibitors?
A novel therapeutic principle emerged from the introduction of prolyl hydroxylase inhibitors, which stabilize hypoxia-inducible factors and subsequently ameliorate anemia by promoting endogenous Epo formation and iron delivery from enterocytes and macrophages. 108 These orally available drugs are being studied in phase 3 clinical trials for the treatment of anemia in hemodialysis, 109 but they could also become useful therapeutic options in AI.
Why do erythrocytes have a shorter lifespan?
A shortened erythrocyte lifespan has been extensively documented in the inflammatory setting and has been attributed to enhanced erythrophagocytosis by hepatic and splenic macrophages caused by “bystander” deposition of antibody and complement on erythrocytes, mechanical damage from fibrin deposition in microvasculature , and activation of macrophages for increased erythrophagocytosis. 23, 46, 47 Shortened erythrocyte survival is usually a minor factor in chronic AI; however, in acute infections, severe sepsis, or other critical illnesses accompanied by a high level of cytokine activation, anemia is detected after hours or a few days (ie, too rapidly to be accounted for by deficient erythropoiesis). It is reasonable that massive erythrophagocytosis, hemolysis, or pooling of erythrocytes, along with hemodilution, contribute to this entity that awaits systematic scientific analysis. 48 Moreover, remediable iatrogenic factors are common in critical illness and include blood loss from phlebotomy and gastrointestinal blood loss caused by nasogastric tubes, anticoagulation, and the use of medications that promote gastroduodenal erosion or ulceration.
What is the most common anemia in hospitalized patients?
Anemia of inflammation ( AI), also known as anemia of chronic disease ( ACD), is regarded as the most frequent anemia in hospitalized and chronically ill patients. It is prevalent in patients with diseases that cause prolonged immune activation, including infection, autoimmune diseases, and cancer. More recently, the list has grown ...
What is the best treatment for AI?
Optimally, the best treatment for AI is cure of the underlying inflammatory disease , which mostly results in resolution of AI. As much as is feasible, the contribution of concomitant pathologies to AI ( Table 2) should also be considered and specifically corrected; however, such fundamental treatments are not always possible or effective. With regard to treatments directed specifically at AI, we lack data from prospective trials about how aggressive such treatments should be or what constitutes the optimal therapeutic end point. Caution is suggested by studies indicating that, in environments with a high endemic burden of infectious diseases, mild anemia and/or iron deficiency may even be beneficial. Infants with mild iron deficiency or anemia were less likely to suffer and die from severe malaria. 81 Indiscriminate dietary iron fortification resulted in increased morbidity and mortality from serious infections, including malaria and enteric infections. 82, 83 Such studies indicate that care should be taken to identify those patients with AI, with or without iron deficiency, who can benefit from iron supplementation or anemia correction. 84 Although not yet widely used, low pretreatment serum hepcidin concentration appears to be a good predictor of therapeutic response to oral iron supplementation, thus potentially avoiding risks to patients who could not benefit from oral iron supplementation. 85
What does it mean when your TIBC is low?
A low TIBC and a normal or elevated ferritin level are the most important signs that indicate that anemia of inflammation is present. With inflammation, the level of certain plasma proteins called acute phase proteins is higher in the blood. The increase in these proteins usually leads to an increase in the blood's sedimentation rate, which is determined by a blood test.
What causes anemia of inflammation?
Although the exact cause of anemia of inflammation is not known, it is related to the effects of chronic inflammatory diseases on the red blood cells. These conditions cause a number of changes in the body's red blood cells. The lifespan of red blood cells becomes shorter, production of new red blood cells in the bone marrow slows down, and iron is "withheld" so that it cannot be used to make new red blood cells. Normally the body recycles iron from "old" red blood cells and uses it to make new ones. In anemia of inflammation, the body does not recycle iron as easily, so it is "held up" in cells such as macrophages (a type of white blood cell). There is also decreased iron absorption from the intestines. These changes are caused by a protein called hepcidin.
What is anemia in health?
Anemia occurs when there aren't enough healthy red blood cells in the blood. Most anemias are more of a symptom than a disease and can be the result of a variety of health conditions. With any form of anemia, it's important to find the cause before treatment begins. Anemia of inflammation, also known as anemia of chronic disease, ...
What are the signs of inflammation?
Accurate diagnosis depends on blood test results such as the following: A low TIBC and a normal or elevated ferritin level are the most important signs that indicate that anemia of inflammation is present. With inflammation, the level of certain plasma proteins called acute phase proteins is higher in the blood.
What are some examples of anemia?
The following are examples of conditions that can cause anemia of inflammation: autoimmune diseases or diseases with inflammation (e.g., rheumatoid arthritis, lupus, ulcerative colitis, Crohn's disease, giant cell [temporal] arteritis)
Why does anemia of inflammation go unnoticed?
Anemia of inflammation may go unnoticed and untreated because the attention is centered on the disease that is causing it. In the past, it was believed that anemia of inflammation was associated only with infections such as syphilis and tuberculosis.
Why is it important to treat anemia?
Once all other causes of anemia are ruled out and the inflammation, infection, or other problem is identified and treated, the anemia may improve.
What is anemia of inflammation?
Anemia of inflammation (AI), also known as anemia of chronic disease (ACD), is regarded as the most frequent anemia in hospitalized and chronically ill patients. It is prevalent in patients with diseases that cause prolonged immune activation, including infection, autoimmune diseases, and cancer. More recently, the list has grown to include chronic kidney disease, congestive heart failure, chronic pulmonary diseases, and obesity. Inflammation-inducible cytokines and the master regulator of iron homeostasis, hepcidin, block intestinal iron absorption and cause iron retention in reticuloendothelial cells, resulting in iron-restricted erythropoiesis. In addition, shortened erythrocyte half-life, suppressed erythropoietin response to anemia, and inhibition of erythroid cell differentiation by inflammatory mediators further contribute to AI in a disease-specific pattern. Although the diagnosis of AI is a diagnosis of exclusion and is supported by characteristic alterations in iron homeostasis, hypoferremia, and hyperferritinemia, the diagnosis of AI patients with coexisting iron deficiency is more difficult. In addition to treatment of the disease underlying AI, the combination of iron therapy and erythropoiesis-stimulating agents can improve anemia in many patients. In the future, emerging therapeutics that antagonize hepcidin function and redistribute endogenous iron for erythropoiesis may offer additional options. However, based on experience with anemia treatment in chronic kidney disease, critical illness, and cancer, finding the appropriate indications for the specific treatment of AI will require improved understanding and a balanced consideration of the contribution of anemia to each patient's morbidity and the impact of anemia treatment on the patient's prognosis in a variety of disease settings.
Where is David Geffen School of Medicine?
3 Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA; and.
Can iron therapy help with anemia?
In addition to treatment of the disease underlying AI, the combination of iron therapy and erythropoiesis-stimulating agents can improve anemia in many patients. In the future, emerging therapeutics that antagonize hepcidin function and redistribute endogenous iron for erythropoiesis may offer additional options.
How to avoid iron deficiency anemia?
But you can avoid iron deficiency anemia and vitamin deficiency anemias by eating a diet that includes a variety of vitamins and minerals, including: Iron. Iron-rich foods include beef and other meats, beans, lentils, iron-fortified cereals, dark green leafy vegetables, and dried fruit. Folate.
What causes low red blood cells?
Vitamin deficiency anemia. Besides iron, your body needs folate and vitamin B-12 to produce enough healthy red blood cells. A diet lacking in these and other key nutrients can cause decreased red blood cell production. Some people who consume enough B-12 aren't able to absorb the vitamin. This can lead to vitamin deficiency anemia, also known as pernicious anemia.
What causes aplastic anemia?
Causes of aplastic anemia include infections, certain medicines, autoimmune diseases and exposure to toxic chemicals. Anemias associated with bone marrow disease. A variety of diseases, such as leukemia and myelofibrosis, can cause anemia by affecting blood production in your bone marrow.
What is the best vitamin for red blood cells?
Besides iron, your body needs folate and vitamin B-12 to produce enough healthy red blood cells. A diet lacking in these and other key nutrients can cause decreased red blood cell production. Also, some people who consume enough B-12 aren't able to absorb the vitamin.
Why do pregnant women have anemia?
Your bone marrow needs iron to make hemoglobin. Without adequate iron, your body can't produce enough hemoglobin for red blood cells. Without iron supplementation , this type of anemia occurs in many pregnant women.
What causes blood loss in the stomach?
It is also caused by blood loss, such as from heavy menstrual bleeding, an ulcer, cancer and regular use of some over-the-counter pain relievers, especially aspirin, which can cause inflammation of the stomach lining resulting in blood loss. Vitamin deficiency anemia.
How to prevent anemia?
Treatments for anemia range from taking supplements to undergoing medical procedures. You might be able to prevent some types of anemia by eating a healthy, varied diet.
What is bone marrow biopsy?
The bone marrow biopsy is performed in an outpatient setting under either local anesthesia or light sedation and involves collecting a sample of bone marrow by inserting a needle into the pelvis. Increased iron stores in the bone marrow, in addition to a low serum iron level, indicate anemia of chronic disease.
Why do people with chronic kidney disease get anemia?
Chronic kidney disease (Nearly every patient with this type of disease will be get anemia because kidneys make erythropoietin (EPO), a hormone that controls the production of red blood cells in the bone marrow.)
Why do red blood cells die?
Chronic diseases may cause changes in red blood cells, the oxygen-carrying blood cells made by bone marrow. These changes can cause red blood cells to die sooner and slow down their production. In anemia of chronic disease, the iron that is normally recycled from old red blood cells to help make new red blood cells is retained within a system ...
How long does anemia last?
Chronic diseases are those that last longer than 3 months.
What is the normal hemoglobin level?
A normal hemoglobin level is 12.3-15.3 g/dL for adult women and 14-17.5 g/dL for adult men. A fingerstick test can be used to measure hemoglobin. Most important, the blood test will reveal a low serum iron in a person with anemia. Serum is a liquid part of blood. The blood test may also reveal:
What are the symptoms of anemia?
Symptoms are similar to those of iron-deficiency anemia and include fatigue, sweating, and headaches. Overview. Symptoms and Causes. Diagnosis and Tests. Management and Treatment. Prevention. Outlook / Prognosis. Anemia of Chronic Disease.
Why are blood transfusions not used as a long term treatment?
Transfusions are not used as a long-term therapy because of risks—such as iron overload and potential immune system side effects— that may increase the risk of getting an infection.
What is it called when your body stops producing blood cells?
Aplastic anemia. Aplastic anemia is when your bone marrow becomes damaged, and your body therefore stops producing new blood cells. It can be sudden or get worse over time. It can also have no known cause, which is referred to as idiopathic aplastic anemia.
What is the condition that causes a lower than normal amount of blood cells?
Fanconi anemia (FA) is a genetic condition that impairs bone marrow and causes you to have a lower than normal amount of all types of blood cells.
What is the cause of aplastic anemia?
It can also have no known cause, which is referred to as idiopathic aplastic anemia.
What is the main symptom of severe malaria?
Malarial anemia is a main symptom of severe malaria. Many factors contribute to its development, including:
Why is anemia dangerous?
In many cases, it’s mild, but anemia can also be serious and life-threatening. Anemia can happen because: Your body doesn’t make enough red blood cells. Bleeding causes you to lose red blood cells more quickly than they can be replaced. Your body destroys red blood cells.
What causes red blood cells to become sickle shaped?
Sickle cell disease. Sickle cell disease is an inherited type of anemia. It causes your red blood cells to be deformed — they become sickle-shaped, rigid, and sticky. This causes them to get stuck in small blood vessels, which blocks blood flow throughout your body, depriving tissue of oxygen.
How are anemias passed down?
These are conditions that cause anemia and are inherited, which means they are passed down through one or both parents through your genes.

Symptoms and Complications
- There is generally a chronic illness or infection present that causes anemia of inflammation, so the symptoms you may experience will vary.The primary condition causing the anemia will have its own set of symptoms. Symptoms of the anemia may include pale skin, lack of energy, fatigue, headache, lethargy (a feeling of "laziness"), shortness of breat...
Making The Diagnosis
- Anemia of inflammation isn't usually severe, and symptoms related to the underlying disease often cover up those of the anemia.This can make it more difficult to diagnose. Accurate diagnosis depends on blood test results such as the following: 1. hemoglobin: low 2. reticulocyte count: low to normal 3. serum ferritin level: normal to elevated 4. serum iron: variable 5. total iro…
Treatment and Prevention
- People with a chronic inflammatory disease should be carefully monitored for anemia so that it can be diagnosed and treated. In most cases, the type of inflammatory disease or tumour that's causing the anemia determines the treatment for anemia of inflammation. It is important to find and treat the underlying cause of the anemia. Once all other causes of anemia are ruled out and …