
Why do insulin receptor substrate proteins need phosphorylation?
Phosphorylation of insulin receptor substrate (IRS) proteins on serine residues has emerged as a key step in these control processes under both physiological and pathological conditions.
Why do we need to phosphorylate glucose?
Phosphorylation of glucose is imperative in processes within the body. For example, phosphorylating glucose is necessary for insulin-dependent mechanistic target of rapamycin pathway activity within the heart. This further suggests a link between intermediary metabolism and cardiac growth.
What is the role of insulin in intracellular metabolism?
One of the important effects of insulin on intracellular metabolism is its ability to stimulate the synthesis of glycogen in muscle and liver. It does this by promoting a net decrease in the extent of phosphorylation of glycogen synthase, the rate-limiting enzyme in the pathway of glycogen synthesis …
How does insulin stimulate the synthesis of glycogen in muscle?
One of the important effects of insulin on intracellular metabolism is its ability to stimulate the synthesis of glycogen in muscle and liver. It does this by promoting a net decrease in the extent of phosphorylation of glycogen synthase, the rate-limiting enzyme in the pathway of glycogen synthesis … How does insulin stimulate glycogen synthesis?
Does insulin phosphorylate glucose?
Insulin activates PI3 kinase and Akt, which increases the uptake of glucose (by translocation of GLUT4 to the cell surface). Once it is phosphorylated, glucose induces mTOR activation. Glucosamine is phosphorylated to glucosamine 6-phosphate, which likely participates in mTOR signalling.
Does insulin phosphorylate GLUT4?
Insulin-induced phosphorylation of AS160 mediates the docking and fusion of GLUT4 vesicles to the plasma membrane.
What is insulin receptor phosphorylation?
Insulin activates the insulin receptor tyrosine kinase (IR), which phosphorylates and recruits different substrate adaptors such as the IRS family of proteins. Tyrosine phosphorylated IRS then displays binding sites for numerous signaling partners.
How does insulin work on GLUT4?
In the absence of insulin, Glut4 slowly recycles between the plasma membrane and vesicular compartments within the cell, where most of the Glut4 resides. Insulin stimulates the translocation of a pool of Glut4 to the plasma membrane, through a process of targeted exocytosis.
How is GLUT4 activated?
The mechanism for GLUT4 is an example of a cascade effect, where binding of a ligand to a membrane receptor amplifies the signal and causes a cellular response. In this case, insulin binds to the insulin receptor in its dimeric form and activates the receptor's tyrosine-kinase domain.
Does insulin phosphorylate AKT?
Upon insulin binding its receptor, Akt is rapidly activated (by the canonical IRS/PI3K pathway) and directly phosphorylates IRS proteins at key regulatory residues such as IRS2 S306 and S577.
How does insulin activate a protein kinase?
When insulin binds to the receptor tyrosine kinase (RTK), it phosphorlylates itself, which then leads to the binding of other proteins to the activated receptor and their phosphorylation. One of these proteins leads to changes in gene transcription.
Does insulin receptor phosphorylate IRS?
The function of insulin receptor substrate-1 (IRS-1) is regulated by both tyrosine and serine/threonine phosphorylation. Phosphorylation of some serine/threonine residues in IRS-1 dampens insulin signaling, whereas phosphorylation of other serine/threonine residues enhances insulin signaling.
What happens to GLUT4 in diabetes?
Glucose transporter 4 (GLUT4) plays a major role in the pathophysiology of T2DM. Its defective expression or translocation to the peripheral cell plasma membrane in T2DM patients hinders the entrance of glucose into the cell for energy production.
How does insulin trigger the uptake of glucose?
In skeletal muscle and adipose tissue, insulin promotes membrane trafficking of the glucose transporter GLUT4 from GLUT4 storage vesicles to the plasma membrane, thereby facilitating the uptake of glucose from the circulation.
How does insulin facilitate the entry of glucose in a cell?
In response, the pancreas secretes insulin, which directs the muscle and fat cells to take in glucose. Cells obtain energy from glucose or convert it to fat for long-term storage. Like a key fits into a lock, insulin binds to receptors on the cell's surface, causing GLUT4 molecules to come to the cell's surface.
Is GLUT4 active or passive transport?
passiveNo, GLUT4 is a passive transporter of glucose down the concentration gradient. It is a glucose transporter present in the adipose tissues, skeletal and cardiac muscles.
How does insulin signaling work?
Insulin signaling at target tissues is essential for growth and development and for normal homeostasis of glucose, fat, and protein metabolism. Control over this process is therefore tightly regulated. It can be achieved by a negative feedback control mechanism whereby downstream components inhibit upstream elements along the insulin-signaling pathway (autoregulation) or by signals from apparently unrelated pathways that inhibit insulin signaling thus leading to insulin resistance. Phosphorylation of insulin receptor substrate (IRS) proteins on serine residues has emerged as a key step in these control processes under both physiological and pathological conditions. The list of IRS kinases implicated in the development of insulin resistance is growing rapidly, concomitant with the list of potential Ser/Thr phosphorylation sites in IRS proteins. Here, we review a range of conditions that activate IRS kinases to phosphorylate IRS proteins on "hot spot" domains. The flexibility vs. specificity features of this reaction is discussed and its characteristic as an "array" phosphorylation is suggested. Finally, its implications on insulin signaling, insulin resistance and type 2 diabetes, an emerging epidemic of the 21st century are outlined.
What is the function of insulin signaling?
Insulin signaling at target tissues is essential for growth and development and for normal homeostasis of glucose, fat, and protein metabolism. Control over this process is therefore tightly regulated. It can be achieved by a negative feedback control mechanism whereby downstream components inhibit …
How does insulin affect the metabolism of the body?
One of the important effects of insulin on intracellular metabolism is its ability to stimulate the synthesis of glycogen in muscle and liver. It does this by promoting a net decrease in the extent of phosphorylation of glycogen synthase, the rate-limiting enzyme in the pathway of glycogen synthesis, which increases its activity.
Does insulin affect glycogen synthesis?
One of the important effects of insulin on intracellular metabolism is its ability to stimulate the synthesis of glycogen in muscle and liver. It does this by promoting a net decrease in ...
How does insulin regulate glucose metabolism?
Insulin governs systemic glucose metabolism, including glycolysis, gluconeogenesis and glycogenesis, through temporal change and absolute concentration. However, how insulin‐signalling pathway selectively regulates glycolysis, gluconeogenesis and glycogenesis remains to be elucidated. To address this issue, we experimentally measured metabolites in glucose metabolism in response to insulin. Step stimulation of insulin induced transient response of glycolysis and glycogenesis, and sustained response of gluconeogenesis and extracellular glucose concentration (GLCex). Based on the experimental results, we constructed a simple computational model that characterises response of insulin‐signalling‐dependent glucose metabolism. The model revealed that the network motifs of glycolysis and glycogenesis pathways constitute a feedforward (FF) with substrate depletion and incoherent feedforward loop (iFFL), respectively, enabling glycolysis and glycogenesis responsive to temporal changes of insulin rather than its absolute concentration. In contrast, the network motifs of gluconeogenesis pathway constituted a FF inhibition, enabling gluconeogenesis responsive to absolute concentration of insulin regardless of its temporal patterns. GLCex was regulated by gluconeogenesis and glycolysis. These results demonstrate the selective control mechanism of glucose metabolism by temporal patterns of insulin. Synopsis The regulation of glucose metabolism by pulse stimulations of insulin is compared with the effect of ramp stimulations. Specific network motifs mediate the differential response to these temporal patterns of stimulations that mimic in vivo patterns of insulin secretion. Temporal patterns and absolute concentration of insulin selectively control glycolysis, gluconeogenesis an Continue reading >>
How does insulin affect muscle?
(3) Protein metabolism: (a) it increases the rate of transport of some amino acids into tissues, (b) it increases the rate of protein synthesis in muscle, adipose tissue, liver, and other tissues, (c) it decreases the rate of protein degradation in muscle (and perhaps other tissues). These insulin effects serve to encourage the synthesis of carbohydrate, fat and protein, therefore, insulin can be considered to be an anabolic hormone. Continue reading >>
What is the enzymatic pathway that converts glucose into pyruvate?
Glycolysis means sugar (glyco) breaking (lysis). It is an enzymatic pathway which converts glucose (a hexose, six carbon sugar) to two molecules of pyruvate (a triose, 3-carbon sugar). Glycolysis occurs in the cytoplasm and does not require the presence of oxygen. It is found (with variations in the terminal steps), in nearly all organisms and is one of the most ancient known metabolic pathways [1]. In aerobic organisms the pyruvate is used to generate more ATP via the citric acid cycle/cytochrome system, or converted into fatty acids and stored as triglycerides. Gluconeogenesis is the reverse, a metabolic pathway that generates glucose from non-carbohydrate carbon substrates such as lactate, all citric acid cycle intermediates (through conversion to oxaloacetate), amino acids other than lysine or leucine, and glycerol. Transamination or deamination of amino acids allows their carbon skeleton to enter the cycle directly (as pyruvate or oxaloacetate), or indirectly via the citric acid cycle. Gluconeogenesis (with glycogenolysis) is one of the two main mechanisms which keep blood glucose levels from dropping too low (hypoglycemia). Gluconeogenesis takes place mainly in the liver and, to a lesser extent, in the cortex of kidneys. Gluconeogenesis occurs during fasting, low-carbohydrate intake or intense exercise, often in association with ketosis. Enzymes involved in glycolysis As shown in Figure 1, there are two phases and ten steps in the glycolytic pathway. The first five steps are regarded as the preparatory phase, since they consume energy to convert the glucose into two three-carbon sugar phosphates [1]. The first step is phosphorylation of glucose by hexokinase to form glucose-6-phosphate. This reaction consumes ATP, but acts to keep the glucose concentration low, prom Continue reading >>
How does diabetes occur?
Diabetes occurs when there is a dis-balance between the demand and production of the hormone insulin. Control of blood sugar When food is taken, it is broken down into smaller components. Sugars and carbohydrates are thus broken down into glucose for the body to utilize them as an energy source. The liver is also able to manufacture glucose. In normal persons the hormone insulin, which is made by the beta cells of the pancreas, regulates how much glucose is in the blood. When there is excess of glucose in blood, insulin stimulates cells to absorb enough glucose from the blood for the energy that they need. Insulin also stimulates the liver to absorb and store any excess glucose that is in the blood. Insulin release is triggered after a meal when there is a rise in blood glucose. When blood glucose levels fall, during exercise for example, insulin levels fall too. High insulin will promote glucose uptake, glycolysis (break down of glucose), and glycogenesis (formation of storage form of glucose called glycogen), as well as uptake and synthesis of amino acids, proteins, and fat. Low insulin will promote gluconeogenesis (breakdown of various substrates to release glucose), glycogenolysis (breakdown of glycogen to release gluose), lipolysis (breakdown of lipids to release glucose), and proteolysis (breakdown of proteins to release glucose). Insulin acts via insulin receptors. Liver Adipose or fat Tissue Muscle High insulin Glycolysis Glycogenesis Triglyceride synthesis Amino acid uptake Protein synthesis Low insulin Gluconeogenesis Glycogenolysis Lipolysis Proteolysis Normal Responses to Eating and Fasting In a fed state: there is increased insulin secretion, causing glycolysis, glycogen storage, fatty acid synthesis/storage, and protein synthesis. After an overnight fast: Continue reading >>
What is the goal of glycogenolysis?
Biosynthesis of Glycogen: The goal of glycolysis, glycogenolysis, and the citric acid cycle is to conserve energy as ATP from the catabolism of carbohydrates. If the cells have sufficient supplies of ATP, then these pathways and cycles are inhibited. Under these conditions of excess ATP, the liver will attempt to convert a variety of excess molecules into glucose and/or glycogen. Glycogenesis: Glycogenesis is the formation of glycogen from glucose. Glycogen is synthesized depending on the demand for glucose and ATP (energy). If both are present in relatively high amounts, then the excess of insulin promotes the glucose conversion into glycogen for storage in liver and muscle cells. In the synthesis of glycogen, one ATP is required per glucose incorporated into the polymeric branched structure of glycogen. actually, glucose-6-phosphate is the cross-roads compound. Glucose-6-phosphate is synthesized directly from glucose or as the end product of gluconeogenesis. Link to: Interactive Glycogenesis (move cursor over arrows) Jim Hardy, Professor of Chemistry, The University of Akron. Glycogenolysis: In glycogenolysis, glycogen stored in the liver and muscles, is converted first to glucose-1- phosphate and then into glucose-6-phosphate. Two hormones which control glycogenolysis are a peptide, glucagon from the pancreas and epinephrine from the adrenal glands. Glucagon is released from the pancreas in response to low blood glucose and epinephrine is released in response to a threat or stress. Both hormones act upon enzymes to stimulate glycogen phosphorylase to begin glycogenolysis and inhibit glycogen synthetase (to stop glycogenesis). Glycogen is a highly branched polymeric structure containing glucose as the basic monomer. First individual glucose molecules are hydrolyzed fr Continue reading >>
How is glucose stored in the body?
Hormonal Control of Glucose The storage and metabolism of glucose is controlled at the organ level largely by hormonal effects . The hormones glucagon and insulin are secreted by the pancreas during periods of low or high blood sugar, respectively. Glucagon causes the liver to produce glucose from the storage polysaccharide glycogen or to synthesize glucose from pyruvate using the pathway gluconeogenesis. The released glucose enters into the blood and travels to muscle for oxidation by glycolysis leading to energy production. In contrast, insulin instructs the liver cells to store the excess glucose in the blood as glycogen within the liver cells. The hormone epinephrine,which is produced by the central nervous system in response to dangerous situations, evokes the same response as glucagon and causes the release of glucose from the liver. Hormonal control of glucose metabolism. Glucagon and insulin are pancreatic hormones that regulate blood sugar levels. Epinephrine (adrenaline) is produced by the adrenal gland in the central nervous system in response to dangerous situations. Glucagon and epinephrine instruct the liver cell to produce glucose by release from glycogen or by synthesis from pyruvate via the synthetic pathway gluconeogenesis. The glucose enters the blood and is oxidized in muscle cells to produce energy. Insulin is secreted when the blood glucose level is high, instructing the liver to store glucose in glycogen or, if necessary, to oxidize it to produce energy. These hormones do not directly affect the enzymes involved in glucose metabolism and storage. Rather they bind to membrane receptors on the surface of the cell and evoke a conformational, or allosteric, change in the receptor, transmitting the signal to the inside of the cell. The hormones glucagon Continue reading >>
What hormones regulate blood glucose levels?
Our discussions of metabolic regulation and hormone action now come together as we return to the hormonal regulation of blood glucose level. The minute-by-minute adjustments that keep the blood glucose level near 4.5 mM involve the combined actions of insulin, glucagon, and epinephrine on metabolic processes in many body tissues, but especially in liver, muscle, and adipose tissue. Insulin signals these tissues that the blood glucose concentration is higher than necessary; as a result, the excess glucose is taken up from the blood into cells and converted to storage compounds, glycogen and triacylglycerols. Glucagon carries the message that blood glucose is too low, and the tissues respond by producing glucose through glycogen breakdown and gluconeogenesis and by oxidizing fats to reduce the use of glucose. Epinephrine is released into the blood to prepare the muscles, lungs, and heart for a burst of activity. Insulin, glucagon, and epinephrine are the primary determinants of the metabolic activities of muscle, liver, and adipose tissue. Epinephrine Signals Impending Activity When an animal is confronted with a stressful situation that requires increased activity-fighting or fleeing, in the extreme case-neuronal signals from the brain trigger the release of epinephrine and norepinephrine from the adrenal medulla. Both hormones increase the rate and strength of the heartbeat and raise the blood pressure, thereby increasing the flow of 02 and fuels to the tissues, and dilate the respiratory passages, facilitating the uptake of O2 (Table 22-3). In its effects on metabolism, epinephrine acts primarily on muscle, adipose tissue, and liver. It activates glycogen phosphorylase and inactivates glycogen synthase (by cAMP-dependent phosphorylation of the enzymes; see Fig. 14-18 a Continue reading >>
How does insulin work in diabetes?
It focuses on the recent discovery of how the hormone insulin actually binds to the receptor on the surface of cells, as determined by Professor Mike Lawrence's laboratory at the Walter and Eliza Hall Institute. Insulin binds to the receptor protein on the cell surface and instructs the cell to take up glucose from the blood for use as an energy source. In type 2 diabetes, we believe that insulin binds to the receptor normally, but the signal is not sent into the cell, the cells do not take up glucose and the resulting high blood glucose levels cause organ damage over time. Understanding how insulin interacts with its receptor is fundamental to the development of novel insulin for the treatment of diabetes. Maja Divjak, 2015 Continue reading >>
How does insulin affect our metabolism?
This is essential for brain function. Regardless of large fluctuations in physical activity and food intake, blood sugar levels are held within very narrow limits. The key to this is insulin, the secretion of which is closely regulated by circulating substrates of energy metabolism. Insulin signals food abundance and initiates uptake and storage of carbohydrates, fats and amino acids. Energy supply and stability of blood sugar levels postprandial is usually accorded to glucagon and the catecholamines, but the reduction in insulin signalling postprandial is almost certainly just as important. How does insulin influence our metabolism? What are the key events in its action? Control of the key enzymes of metabolism can be divided into two classes: 1. Covalent modification of enzymes, usually by phosphorylation or dephosphorylation of serine, threonine or tyrosine residues. 2. Allosteric feedback and feed-forward regulation by metabolic intermediates. Enzymes involved in metabolism can be either activated or inactivated by phosphorylation. Examples or this are glycogen phosphorylase and hormone-sensitive lipase which are activated when phosphorylated and glycogen synthetase and pyruvate dehydrogenase are inactivated through phosphorylation. The protein kinases that catalyze phosphorylation of these enzymes are subject to control through cyclic nucleotides (PKA and cyclic AMP), Ca++ and diacylglycerol (PKC) and PI (3,4,5P)P3 (PKB). The extent of enzyme phosphorylation is controlled by the balance between protein kinases and protein phosphatases. The picture becomes extremely complex when one knows that protein kinases can act Continue reading >>
How does the cellular receptor for insulin work?
The cellular receptor for insulin helps control the utilization of glucose by cells Cells throughout the body are fueled largely by glucose that is delivered through the bloodstream . A complex signaling system is used to control the process, ensuring that glucose is delivered when needed and stored when there is a surplus. Two hormones, insulin and glucagon, are at the center of this signaling system. When blood glucose levels drop, alpha cells in the pancreas release glucagon, which then stimulates liver cells to release glucose into the circulation. When blood glucose levels rise, on the other hand, beta cells in the pancreas release insulin, which promotes uptake of glucose for metabolism and storage. Both hormones are small proteins that are recognized by receptors on the surface of cells. Signal Transduction The receptor for insulin is a large protein that binds to insulin and passes its message into the cell. It has several functional parts. Two copies of the protein chains come together on the outside of the cell to form the receptor site that binds to insulin. This is connected through the membrane to two tyrosine kinases, shown here at the bottom. When insulin is not present, they are held in a constrained position, but when insulin binds, these constraints are released. They first phosphorylate and activate each other, and then phosphorylate other proteins in the signaling network inside the cell. Since the whole receptor is so flexible, researchers have determined its structure in several pieces: the insulin-binding portion is shown here from PDB entry 3loh , the transmembrane segment from 2mfr , and the tyrosine kinase from 1irk . When Things Go Wrong Problems with insulin signaling can impair the proper management of glucose levels in the blood, leading to Continue reading >>
How does insulin interact with the IR?
When Insulin interacts with the , a part of the Insulin Receptor (IR), it triggers a phosphorylation cascade starting in the holoreceptor's tyrosine kinase domain. This leads to the introduction of glucose into the cell. Without Insulin, glucose is prevented from entering the cell; thus, Insulin's interaction with the IR regulates the intracellular concentration of glucose. Until recently, the Insulin-IR binding mechanism was unknown. We will uncover the newly discovered mechanisms between Insulin and its receptor by highlighting the interactions that solidify its binding. II. General Structure Turn spin on/off Insulin is stored as a zinc-coordinated hexamer. However, this hexamer dissociates into zinc-free monomers that are able to bind to the IR. A single Insulin monomer has two chains - and - that are connected by three disulfide bonds (Figure 1), one of which is an intramolecular disulfide bond on Chain A. Both of these chains are needed for Insulin to interact with its receptor. Figure 1: Disulfide Bonds in an Insulin Monomer The Insulin receptor is a heterotetramer consisting of multiple subunits. Each IR monomer includes an alpha-subunit leucine-rich repeat domain (L1 Beta Two Sheet) combined with a cysteine-rich domain (CR) , as well as an alpha-subunit C-terminal segment (alpha-CT) . These are located in the extracellular matrix and constitute the . The supplementary image shows an additional leucine-rich repeat domain (L2) and the first, second, and third fibronectin type III domains, which, combined with the microreceptor, constitute the holoreceptor. There are two isoforms of th Continue reading >>
What is the IR of insulin?
The insulin receptor (IR) is a large, disulphide-linked, glycoprotein that spans the cell membrane with its insulin binding surfaces on the outside of the cell and its tyrosine kinase domains on the inside. IR is a symmetrical homodimer that contains two identical binding pockets, each created by the juxtapositioning of two distinct binding sites involving residues from both IR monomers (IR and IR´). The two binding pockets comprise site 1/site 2´ on one side of IR and site 1´ and site 2 on the opposite side. The current model for IR activation is that two distinct surfaces of insulin engage sequentially with either the site 1/site 2´ binding pocket or the site 1´/site 2 pocket. The formation of a site 1 – insulin - site 2 high-affinity, cross-link involves structural changes in both insulin and IR resulting in the activation of the intracellular tyrosine kinase and the initiation of the phosphorylation cascades that drive insulin signaling. Understanding how insulin binding induces signal transduction requires structures of: (i) insulin and IR in their basal states, (ii) insulin bound to the IR ectodomain, (iii) the activated IR kinase domain and (iv) the domain rearrangements associated with the formation of the high affinity insulin/IR complex that initiates activation of the intracellular kinase. Continue reading >>
Where is insulin secreted?
Go to: ABSTRACT Insulin is an anabolic peptide hormone secreted by the b cells of the pancreas acting through a receptor located in the membrane of target cells - major ones being liver (where it promotes glucose storage into glycogen and decreases glucose output), as well as skeletal muscle and fat (where it stimulates glucose transport through translocation of GLUT4), but also b cells, brain cells and in fact most cells, where it has pleiotropic effects. The receptor belongs to the receptor tyrosine kinase superfamily and has orthologues in all metazoans. The structure of the unbound extracellular domain ("apo-receptor") has been solved. Insulin binds to two distinct sites on each a subunit of the receptor, crosslinking the two receptor halves to create high affinity. The structure of the site 1 interface has also been solved, as well as the structure of the inactive and activated tyrosine kinase, revealing the activation by phosphorylation of an autoinhibitory loop. The receptor activates a complex intracellular signaling network through IRS proteins and the canonical PI3K and ERK cascades. Overall and tissue-specific targeted gene disruption in mice has explored the role of many of the signaling proteins in creating the type 2 diabetes phenotype, with some surprising results. Insulin signaling in the liver and b cell is emerging as the major determinant in preventing type 2 diabetes, through the integrative role of molecules like IRS2 and FOXO, preventing b cell dedifferentiation. The emerging new biology of diabetes opens novel therapeutic opportunities for the 442 million type 2 diabetics worldwide. For complete coverage of this and all related areas of Endocrinology, please visit our FREE on-line web-textbook, www.endotext.org. Go to: INTRODUCTION Insulin is an a Continue reading >>
Does insulin bind to fibroblasts?
Insulin binding, internalization, and insulin receptor regulation in fibroblasts from type II, non-insulin-dependent diabetic subjects. The ability of insulin to bind, internalize, and regulate its own receptor wasinvestigated in cultured human fibroblasts obtained from 8 normal subjects and 8 patients with type II, non-insulin-dependent diabetes mellitus (NIDDM). Theability of the cells from the two groups to bind insulin was the same, andScatchard analysis demonstrated identical curvilinear plots. When cells wereincubated at 37 degrees C with the lysosomotropic agent, chloroquine, and125I-insulin, the drug led to a marked, but comparable, increase incell-associated radioactivity in both control and diabetic fibroblasts (236 and245% increase, respectively). Insulin pretreatment leads to a loss of insulinreceptors in cultured human fibroblasts and preincubation with insulin led to acomparable dose-dependent decrease in subsequent insulin binding in both normaland diabetic fibroblasts. Scatchard analysis demonstrated that this decrease inbinding was entirely due to a decrease in receptor number with no change inreceptor affinity. These data demonstrate normal insulin binding, insulininternalization, and insulin-mediated receptor loss in fibroblasts from patients with with NIDDM, and these cells are several generations removed form the in vivomilieu. Thus, these results provide direct evidence that the well-known decrease in insulin binding in freshly isolated cells from patients with NIDDM, and these cells are several generations removed from the in vivo milieu. Thus, theseresults provide direct evidence that the well-known decrease in insulin bindingin freshly isolated cells form patients with NIDDM is a reflection ofenvironmental factors rather than an intrinsic (gene Continue reading >>
What is the role of insulin in the cell?
A key action of insulin is to stimulate glucose uptake into cells by inducing translocation of the glucose transporter, GLUT4, from intracellular storage to the plasma membrane.
How does insulin affect the liver?
In addition to promoting glucose storage, insulin inhibits the production and release of glucose by the liver by blocking gluconeogenesis and glycogenolysis (Saltiel and Kahn 2001). Insulin directly controls the activities of a set of metabolic enzymes by phosphorylation and dephosphorylation events and also regulates the expression ...
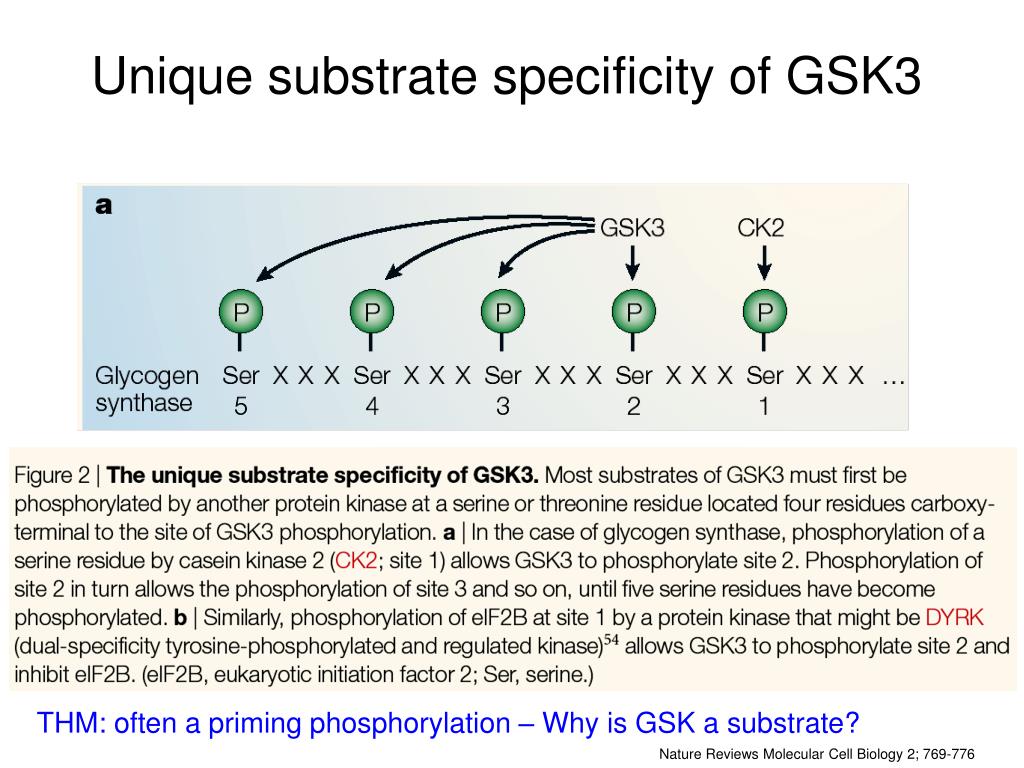
What is the substrate of GSK3?
A major substrate of GSK3 is glycogen synthase, an enzyme that catalyzes the final step in glycogen synthesis. Phosphorylation of glycogen synthase by GSK3 inhibits glycogen synthesis; therefore the inactivation of GSK3 by AKT promotes glucose storage as glycogen. In addition to promoting glucose storage, insulin inhibits ...
What is the catalytic subunit of PI3-kinase?
The catalytic subunit of PI3-kinase, p110, then phosphorylates phosphatidylinositol (4,5) bisphosphate [ PtdIns (4,5) P 2] leading to the formation of Ptd (3,4,5)P3. A key downstream effector of Ptd (3,4,5)P3 is AKT, which is recruited to the plasma membrane. Activation of AKT also requires the protein kinase 3-phosphoinositide dependent protein kinase-1 (PDK1), which in combination with an as yet unidentified kinase leads to the phosphorylation of AKT (Figure 2).
Why is insulin resistant in type 2 diabetes?
Type-1 diabetes is characterized by the inability to synthesize insulin, whereas in type-2 diabetes the body becomes resistant to the effects of insulin, presumably because of defects in the insulin signaling pathway. View our interactive insulin signaling pathway.
What is the phosphorylation of CBL?
In this pathway, insulin receptor activation leads to the phosphorylation of Cbl, which is associated with the adaptor protein CAP. Following phosphorylation the Cbl-CAP complex translocates to lipid rafts in the plasma membrane.
What hormone is released by pancreatic beta cells in response to elevated levels of nutrients in the blood?
By Claudie Hooper, PhD. Introduction. Insulin is a hormone released by pancreatic beta cells in response to elevated levels of nutrients in the blood. Insulin triggers the uptake of glucose, fatty acids and amino acids into liver, adipose tissue and muscle and promotes the storage of these nutrients in the form of glycogen, ...
