Is iodoform the same as iodine?
Ans: Iodoform is not the same as iodine. Iodoform is an organoiodine compound with the formula CH I 3 and has a tetrahedral molecular geometry. Iodine, on the other hand, is a simple molecule. Q.3. What is the iodoform packing strip used for?
Which compounds give positive iodoform test?
Compounds That Give Positive Iodoform Test 1 Acetaldehyde 2 Methyl Ketones 3 Ethanol 4 Secondary Alcohols that contain Methyl Groups in Alpha Position More ...
How do you convert iodoform to diiodomethane?
Some reagents (e.g. hydrogen iodide) convert iodoform to diiodomethane. Also conversion to carbon dioxide is possible: Iodoform reacts with aqueous silver nitrate to produce carbon monoxide. When treated with powdered elemental silver the iodoform is reduced, producing acetylene.
See more

What is iodoform made up of?
First prepared in 1822, iodoform is manufactured by electrolysis of aqueous solutions containing acetone, inorganic iodides, and sodium carbonate.
What does iodoform do for wounds?
Iodoform gauze removes necrotic tissue from pressure ulcer wounds by fibrinolytic activity.
Is iodoform harmful to humans?
* Iodoform can affect you when breathed in and by passing through your skin. * Contact can irritate the skin and eyes. * Breathing Iodoform can irritate the nose and throat. * Exposure to high levels can affect the nervous system causing confusion, irritability, headache, hallucinations and/or poor muscle coordination.
What elements do you think are present in iodoform?
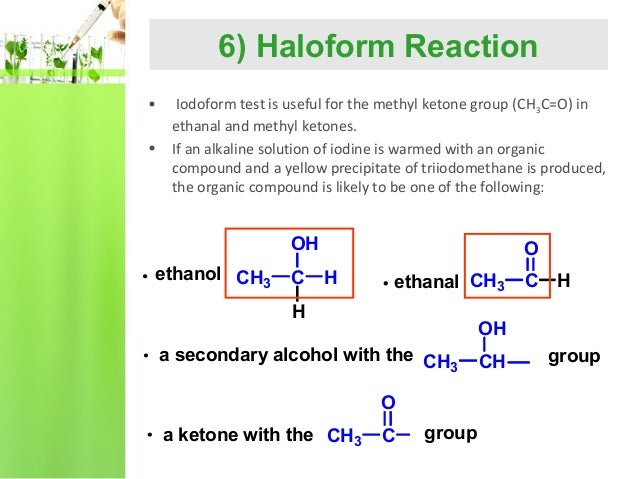
The iodoform reaction identifies a CH2CH2 (OH) group in alcohols. When you add iodine and sodium hydroxide to a compound that contains either a methyl ketone or secondary alcohol with a methyl group in the alpha position, there is the formation of a pale yellow precipitate of iodoform.
How long can you leave iodoform in a wound?
A small wick of iodoform gauze can be placed in the cavity for 24 hours. Many clinicians use antibiotics in patients with felons to cover for MRSA.
Is iodoform good for open wounds?
Iodoform gauze packing strips from Integrity Medical are a sterile single-use wound dressing consisting of a single cotton gauze strip impregnated with formulated Iodoform solution that are packaged in HDPE amber colored jars. They are primarily used for sterile drainage of open and/or infected wounds.
Is iodoform a cytotoxic?
Conclusions: Direct and indirect exposure to high concentrations of iodoform-containing root canal filling material showed a cytotoxic effect on macrophages and epithelial cells, while low concentrations induced cell proliferation.
What does iodoform smell like?
It has a penetrating and distinctive odour with a sweetish smell which is analogous to chloroform. It was widely used in hospitals and described as smell of hospital due to its distinctive odour. Iodoform is commonly used as a disinfectant and antiseptic component of some medications due to its nonirritant action.
How do you pack a wound with iodoform?
1:535:08Wound Management Home Skills Program: Packing a WoundYouTubeStart of suggested clipEnd of suggested clipUse a cotton swab to gently push the packing material into the wound and filled the wound. Space.MoreUse a cotton swab to gently push the packing material into the wound and filled the wound. Space. Leave a small tail of packing material out of the package.
What are the physical properties of iodoform?
IodoformNamesAppearancePale, light yellow, opaque crystalsOdorSaffron-likeDensity4.008 g cm−3Melting point119 °C (246 °F; 392 K)61 more rows
How do you make iodoform?
Preparation of Iodoform Procedure: • Dissolve 5 g of iodine in 5 ml acetone in a conical flask. discharged. Allow contents of flask to stand for 10 – 15 minutes. Filter the yellow precipitate of iodoform through Buchner funnel • Wash the precipitate with cold water.
What gives a positive result in the iodoform test?
Using iodine and sodium hydroxide solution A positive result is the appearance of a very pale yellow precipitate of triiodomethane (previously known as iodoform) - CHI3. Apart from its color, this can be recognised by its faintly "medical" smell.
Q.1. Which compounds give the iodoform test?
Ans: Following compounds give the Iodoform test; a. Ethanol b. Propan–2–ol c. Lactic acid d. Acetaldehyde e. Methyl ketones
Q.2. Is Iodoform the same as iodine?
Ans: Iodoform is not the same as iodine. Iodoform is an organoiodine compound with the formula CHI3 and has a tetrahedral molecular geometry. Iodin...
Q.3. What is the iodoform packing strip used for?
Ans: Iodoform packing strips are used for sterile drainage open and infected wounds.
Q.4. Why does Iodoform show antiseptic properties?
Ans: Iodoform shows antiseptic properties due to the free iodine that Iodoform liberates.
Q.5. Describe the structure of Iodoform.
Ans: The iodoform molecule contains 4 bonds, of which 3 are C–I bonds, and one is a C–H bond.
How is iodoform produced?
Iodoform is produced by heating ethanol with iodine in the presence of alkali ( NaOH or N a 2 C O 3) at about 60 degrees Celsius. When NaOH is used as an alkali, the reaction proceeds as follows.
What is iodoform used for?
Iodoform is a pale yellow, crystalline, volatile substance. It has long been used in general medicine as external disinfection and wound dressing. However, it is no longer employed for specific reasons.
What is the reaction of iodine and haloform?
Iodoform Reaction (Haloform Reaction) The haloform reaction occurs when a methyl ketone reacts with iodine in the presence of hydroxide ions, producing a carboxylate ion and a haloform. Acetaldehyde is the only aldehyde that undergoes the haloform reaction.
What is the name of the compound that is a crystalline yellow substance?
Iodoform is an organic iodine chemical with the formula CHl3 and is a member of the organic halogen family. It is a crystalline pale-yellow material that is highly flammable. Iodoform is also known as triiodomethane. In addition, it is commonly known as the triiodo derivative of methane.
How many bonds does an iodoform have?
Structure of Iodoform. Iodoform has a tetrahedral molecular geometry. The iodoform molecule contains 4 bonds , of which 3 are C– I bonds, and one is a C– H bond.
When was iodoform invented?
Iodoform was invented in 1822. Electrolysis of aqueous solutions containing acetone, inorganic iodides, and sodium carbonate is used to make it. Iodoform is a pale yellow, crystalline, volatile substance. It has long been used in general medicine as external disinfection and wound dressing. However, it is no longer employed for specific reasons. It is an antiseptic component used in medications to treat minor skin diseases; its antiseptic properties were discovered in 1880. To know more about iodoform, read the below article.
What are the physical characteristics of an iodoform?
The following are the important physical features of the Iodoform. It is a yellow crystalline solid with a melting point of 121 degrees Celsius. It has a distinct and unpleasant odour. It is insoluble in water but soluble in ethyl alcohol and ether.
What is the iodine transfer copolymerization of MMA?
Iodine transfer copolymerization of MMA with styrene was performed in bulk at 60 °C in the presence of iodoacetonitrile or iodoform as transfer agents and Et2 AlCl as Lewis acid, which reduces the electron density of the double bond of MMA ( Scheme 23 a). However, this system was not investigated in detail and such studies would deserve more attention. 156
Is iodine a weak oxidizing agent?
Its disinfectant properties may be due to reactions with olefins, and thus to unsaturated lipids, interfering with cell membranes as for iodine, but it is also a weak oxidising agent and so can affect many other cellular and microbial functions. Some reactions may release iodine, although this is not by hydrolysis.
Is bismuth iodoform paraffin paste neurotoxic?
Upon examinations, her serum bismuth level was elevated to 391 nM, and BIPP was removed. Two days after the removal, the symptoms resolved; however, her serum bismuth level remained elevated at 120 nM. Bismuth iodoform paraffin paste has been associated with neurotoxicity presenting with muscle spasm, tremor, confusion, ataxia, memory impairment, clinical depression, acute liver and renal dysfunction, methaemoglobinaemia, and incontinence. Enteropathy also has been reported with BIPP use in dura mater or oral mucosa.
What is iodoform used for?
Due to its antimicrobial properties following topical administration, minimal levels of iodoform may be found in disinfectants and it is more primarily used for veterinary purposes. Iodoform has also been found in dental paste and root canal filling materials in combination with other intracanal medications due to its radiopacity 2. Since the beginning of the 20th century, iodoform has been commonly used as a healing and antiseptic dressing or powder for wounds and sores, however such clinical use to this date is limited. Iodoform is soluble in fatty acids and decomposes releasing iodine in nascent state (96,7% of iodine) when in contact with secretions or endodontic infections 2.
Where is iodoform absorbed?
Iodoform is reported to be absorbed through denuded skin, wounds or mucous membranes 6.
What is the mechanism of action of iodoform?
While the mechanism of action of iodoform remains unclear, it is proposed that iodoform releases iodine, which denatures bacterial proteins by oxidation of the free iodine 5. Iodoform may also play a role in chemical debridement for effective necrotic wound healing and tissue damage repair via collagen fibrinolysis; upon treatment in necrotic tissue, iodoform reduced the size of the macromolecules containing collagen I in wound surface proteins 3. In human gingival fibroblasts in vitro, high concentrations of iodoform was shown to decrease the viability of macrophages and epithelial cells and reduced the secretion of P. gingivalis -induced TNFα 4. P. gingivalis is an anaerobic bacteria present in anaerobic oral niches including periapical sites and periodontal pockets 4.
Does iodoform gauze work?
Iodoform exhibits antibacterial activities after topical application. In a comparative study of wound dressing agents, iodoform gauze exerted an antibacterial effect 3 hours after the start of bacterial growth of E. coli and subsequently maintained the strong antibacterial effectiveness 5.
Does iodoform affect cell proliferation?
A study demonstrated that direct and indirect exposure to high concentrations of iodoform induces a cytotoxic effect on cultures of macrophages and epithelial cells in vitro, while cell proliferation was enhanced at low concentrations of iodoform 4.
Is iodoform a dressing?
Since the beginning of the 20th century, iodoform has been commonly used as a healing and antiseptic dressing or powder for wounds and sores, however such clinical use to this date is limited.
Does iodoform cause hepatocellular damage?
Hepatocellular damage has been observed with iodoform toxicity, where production of both fatty liver and necrosis was seen, as well as lesions of liver membranous cellular components 6. Iodoform induces hepatocellular injury and hepatic lesions in a similar manner to carbon tetrachloride, where cellular membranes may possibly be primary sites of action 1.
Incision and Drainage of an Abscess
Grant C. Fowler MD, in Pfenninger and Fowler's Procedures for Primary Care, 2020
Incision and Drainage
James R. Roberts MD, FACEP, FAAEM, FACMT, in Roberts and Hedges’ Clinical Procedures in Emergency Medicine and Acute Care, 2019
Incision and Drainage of an Abscess
Patrick C. Auth, George S. Bottomley, in Essential Clinical Procedures (Second Edition), 2007
Common Infections of the Hand
Terri M. Skirven OTR/L, CHT, in Rehabilitation of the Hand and Upper Extremity, 2021
Non-surgical root-canal treatment
These include sodium hypochlorite, iodine-potassium-iodide, and a combination of calcium hydroxide and iodoform preparations (Vitapex®, Metapex®, Forendo®).
Chain Polymerization of Vinyl Monomers
Iodine transfer copolymerization of MMA with styrene was performed in bulk at 60 °C in the presence of iodoacetonitrile or iodoform as transfer agents and Et2 AlCl as Lewis acid, which reduces the electron density of the double bond of MMA ( Scheme 23 a).
Pulp Therapy for the Primary Dentition
Anna B. Fuks, ... Brian D. Hodgson, in Pediatric Dentistry (Sixth Edition), 2019
Overdose
If someone has overdosed and has serious symptoms such as passing out or trouble breathing , call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center.
Images
This survey is being conducted by the WebMD marketing sciences department.

Identification
- Generic Name
1. Iodoform - DrugBank Accession Number
1. DB13813
Pharmacology
- Indication
1. No approved therapeutic indications. Reduce drug development failure ratesBuild, train, & validate machine-learning models with evidence-based and structured datasets.See howsee_moreBuild, train, & validate predictive machine-learning models with structured dataset… - Contraindications & Blackbox Warnings
1. Avoid life-threatening adverse drug eventsImprove clinical decision support with information on contraindications & blackbox warnings, population restrictions, harmful risks, & more.Learn moresee_moreAvoid life-threatening adverse drug events & improve clinical decision support.Le…
Interactions
- Drug Interactions information
1. This information should not be interpreted without the help of a healthcare provider. If you believe you are experiencing an interaction, contact a healthcare provider immediately. The absence of an interaction does not necessarily mean no interactions exist. Not Available - Food Interactions
1. Not Available
Categories
- ATC Codes
1. D09AA13 — Iodoform
Chemical Identifiers
- UNII
1. KXI2J76489 - CAS number
1. 75-47-8
Pharmacoeconomics
- Manufacturers
1. Not Available - Packagers
1. Not Available
Properties
- State
1. Solid
Spectra
- Mass Spec (NIST)
1. Not Available