What happens when iron reacts with dilute hydrochloric acid?
Iron reacts with dilute hydrochloric acid to form iron chloride and hydrogen gas. Iron has virtually has no reaction with cold water. However, when both water and oxygen are present (moist air), iron corrodes.
What happens when iron is mixed with acid?
Then, how does iron react with acid? Iron reacts with dilute hydrochloric acid to form iron chloride and hydrogen gas. Iron has virtually has no reaction with cold water. However, when both water and oxygen are present (moist air), iron corrodes.
What happens when sulfuric acid reacts with iron?
When sulfuric acid reacts with iron it makes iron (II) sulfate which is a pale green color. Unfortunately, iron (II) is not stable, it disproportionates into iron (0) and iron (III) by the following reaction.
What happens when a metal reacts with dilute acid?
When a metal reacts with dilute acid, salts are formed. During this reaction hydrogen gas is evolved. In other words, when a metal is added to dilute acids, salt and hydrogen gas are formed. Sulfate salts and chloride salts are formed when metals react with dilute sulfuric acid and hydrochloric acid.
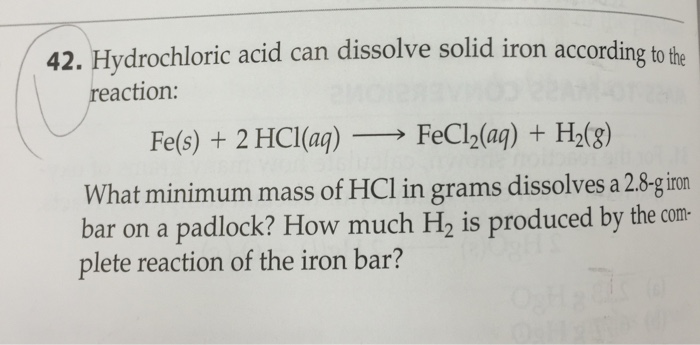
Can iron metal react with acid?
For the acids use hydrochloric acid, nitric acid and sulfuric acid. For the metals use magnesium, zinc and iron....How many different salts can you make using your six cards?MetalAcidSalt nameZincSulfuric acidZinc sulfateIronHydrochloric acidIron chloride7 more rows
Does iron react with acid or base?
3:294:57Reactions of Iron | Reactions | Chemistry | FuseSchool - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe correct answer is that iron will displace any of the metals from a solution of its ions.MoreThe correct answer is that iron will displace any of the metals from a solution of its ions.
Does iron not react with dilute hydrochloric acid?
Iron is the metal highly reactive with dilute hydrochloric acid because it is more active than the hydrogen in the activity series. So, option C is incorrect. - Therefore the metals which do not react with dilute hydrochloric acid are copper and mercury.
Does iron react slowly with acid?
The choice of acid is usually hydrochloric acid of concentration 1 mol dm-3 but some metals react better with sulfuric acid; e.g. zinc. Iron reacts but only very slowly.
What does iron react with?
Chemical properties of iron Iron enters into a reaction with substances of different classes, and interacts with oxygen, carbon, phosphorus, halogens (bromine, iodine, fluorine and chlorine), and also nitrogen. These are not all the reactions of iron – this metal reacts with many elements.
Does iron oxidize in acid?
For example, you form Fe2O3 on Fe (when talking about iron or steel). Acid can dissolve the rust (Fe2O3). However, the acid will also oxidize the metal further, forming more rust, and dissolving that rust. In the simplest case, lets imagine hydrochloric acid and iron (HCl and Fe).
Which metals do not react with dilute acid?
As a result, copper, silver and mercury cannot react with the dilute acid as they won't displace the hydrogen from non-metal anion. Hence, Option D is correct.
Does HCl react with iron?
The reaction between iron and hydrochloric acid The chloride formed when iron reacts with hydrochloric acid is iron (II) chloride, also known as iron dichloride. The reaction between iron and hydrochloric acid is slower than the reaction with zinc, with much smaller hydrogen bubbles produced.
Which metals will not react with dilute hydrochloric acid?
Copper and mercury metal do not react with dilute hydrochloric acid as they come after hydrogen in the activity series, i.e., they can't replace hydrogen from hydrochloric acid.
Which metals react with dilute acids?
Dilute acids react with relatively reactive metals such as magnesium, aluminium, zinc and iron. The products of the reaction are a salt plus hydrogen gas. Here's a good way to remember it: MASH (M+A→S+H). In general, the more reactive the metal, the faster the reaction.
Do all metals react with acid?
Answer: Acids react with most metals to form hydrogen gas and salt. Nevertheless, not all metals react in the same way to acids.
Which metal is most reactive with acid?
7.3 The Relative Activity of MetalsMETALWATERHClsodiumHighly reactive in water.Highly reactive in acid.magnesiumNo reaction in water at room temperature.Reacts rapidly in acid.aluminumNo reaction in water at room temperature.No reaction.ironNo reaction in water.No reaction.