Lithium aluminium hydride (LiAlH4
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula LiAlH₄. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, …
Ketone
In chemistry, a ketone is a functional group with the structure RC(=O)R', where R and R' can be a variety of carbon-containing substituents. Ketones and aldehydes are simple compounds that contain a carbonyl group. They are considered "simple" because they do not have reactive groups like −O…
How can I reduce amides with LiAlH4?
Amides, RCONR'2, can be reduced to the amine, RCH2NR'2 by conversion of the C=O to - CH2 - Amides can be reduced by LiAlH 4 but NOT the less reactive NaBH 4 Typical reagents : LiAlH 4 / ether solvent followedby aqueous work-up.
Is NaBH4 a good reducing agent?
NaBH4 (Sodium borohydride) is a good reducing agent. Although not as powerful as lithium aluminum hydride (LiAlH4), it is very effective for the reduction of aldehydes and ketones to alcohols. By itself, it will generally not reduce esters, carboxylic acids, or amides (although it w
How do you reduce amides to amines?
Amides, RCONR'2, can be reduced to the amine, RCH2NR'2 by conversion of the C=O to - CH2 - Amides can be reduced by LiAlH 4 but NOT the less reactive NaBH 4
Why is LiAlH4 used in dry solvents?
The hydride ion in LiAlH4 is very basic. For this reason, LiAlH4 reacts violently with water and therefore must be used in dry solvents such as anhydrous ether and THF. … The lithium ion acts as a Lewis acid catalyst by coordinating to the carbonyl oxygen.
What does LiAlH4 do to amines?
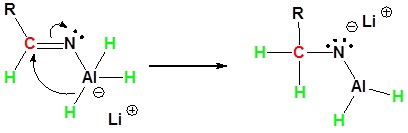
Nitriles can be converted to 1° amines by reaction with LiAlH4. During this reaction the hydride nucleophile attacks the electrophilic carbon in the nitrile to form an imine anion. Once stabilized by a Lewis acid-base complexation the imine salt can accept a second hydride to form a dianion.
What can LiAlH4 not reduce?
LiAlH4 is a mild oxidising agent which can reduce upto alcohol only, it can't reduce any compounds to alkanes.
Can LiAlH4 reduce amide to amine?
Description: Amides can be reduced to amines with a strong reducing agent like lithium aluminum hydride (LiAlH4).
Which functional group Cannot be reduced by LiAlH4?
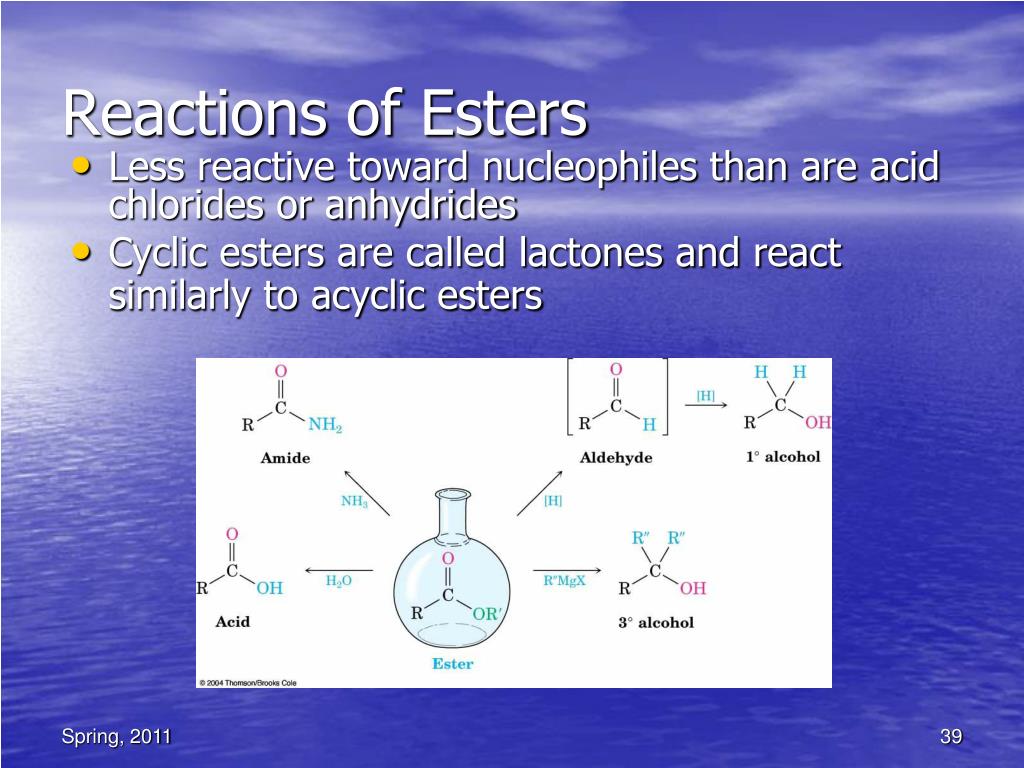
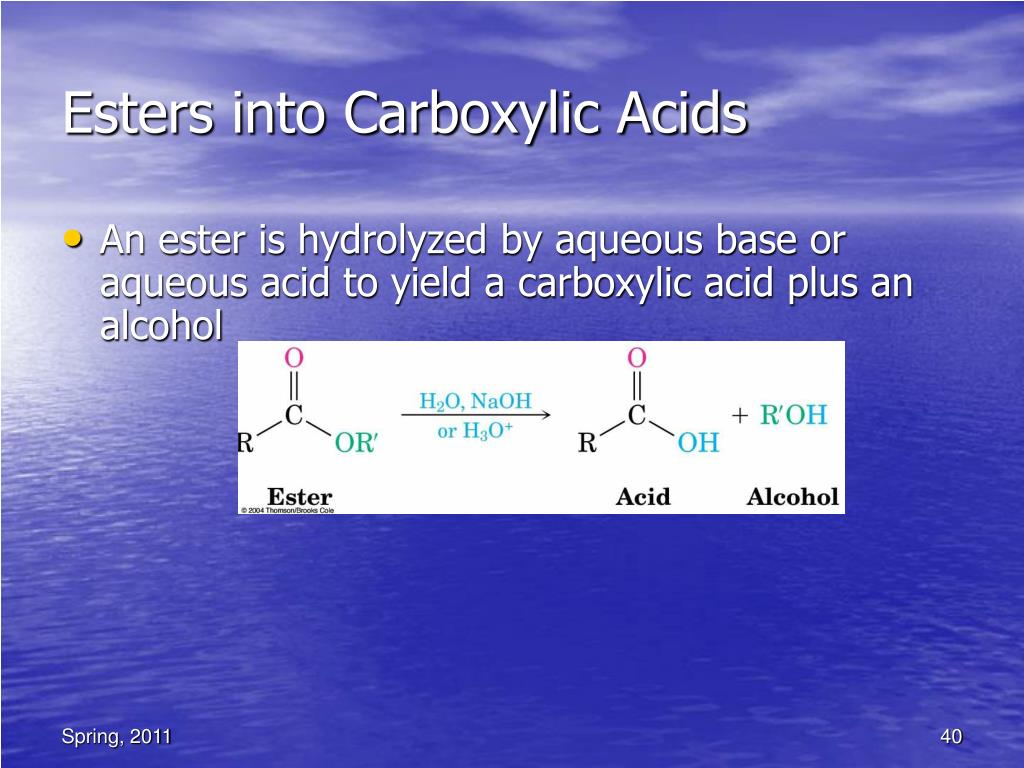
* LiAlH4 reagent can reduce aldehydes to primary alcohols, ketones to secondary alcohols, carboxylic acids and esters to primary alcohols, amides and nitriles to amines, epoxides to alcohols and lactones to diols. * Lithium aluminium hydride, LAH reagent cannot reduce an isolated non-polar multiple bond like C=C.
Which compound does not react with LiAlH4?
12 The compound which does not react with lithiun aluminium hydride is Alpenten-2-one B)
Can LAH reduce no2 to nh2?
The answer is No. LAH is a nucleophilic reductant providing H- ions that reacts with electrophiles.
How can we reduce amines?
Catalytic hydrogenation can be used to reduce amides to amines; however, the process often requires high hydrogenation pressures and reaction temperatures to be effective (i.e. often requiring pressures above 197 atm and temperatures exceeding 200 °C).
How do you convert amide into amine?
When primary amide is treated with an aqueous solution of KOH and bromine, it gives a primary amine.
What happens when amide reacts with LiAlH4?
Amides on reduction with LiAlH4 form primary amines.
Why is LiAlH4 a good reducing agent?
LiAlH4 is a strong reducing agent because aluminium is less electronegative, and the Al-H bond in LiAlH4 is more polar.
Can NaBH4 reduce amides?
When used alone, NaBH4 reduces aldehydes, ketones, acid chlorides, and in some cases esters, but not carboxylic acids, amides, nitriles, nitro compounds or halogenated organic molecules.
Why can't LiAlH4 reduce alcohol?
The main issue is that the Al needs to remove its hydride. With a carboxylic acid and/or an aldehyde, it can stick its hydride onto the carbonyl carbon without issue. But the carbon bonded to the alcohol cannot take on a hydride.
Can LiAlH4 reduce aromatic?
Lithium aluminum hydride (LiAlH4) represents a very versatile reducing agent that is extremely useful in synthetic organic chemistry. It is a more powerful reducing agent than sodium borohydride and reduces aromatic nitro compounds affording their corresponding azo compounds.
Can LiAlH4 reduce a double bond?
Abstract. The reduction of secondary allyl amides with LiAlH4 can lead to a concomitant reduction of the double bond. Previously, an excess of LiAlH4 in hazardous solvents was used for the reduction.
Can LiAlH4 reduce alkyl halides?
We learn the reduction of Alkyl Halides to Alkanes by using 'LiAlH4'. Moreover, Frankland Reagent is also discussed along with a few important examples.
Why NaBH4 Cannot reduce carboxylic acids?
Note that NaBH4 is not strong enough to convert carboxylic acids or esters to alcohols. An aldehyde is produced as an intermediate during this reaction, but it cannot be isolated because it is more reactive than the original carboxylic acid. ) is not a strong enough reducing agent to perform this reaction.
What is Ch20 reduction?
Ch20: Reduction of Amides using LiAlH4 to amines
Is R an alkyl or aryl?
R, R'or R"may be either al kyl or aryl substituents.