
Why does ninhydrin react with proline but not with amino acids?
Apr 04, 2020 · Does ninhydrin react with proline? The nitrogen is the only portion of the purple anion that is derived from the amino acid. Proline and hydroxy-proline do not react with ninhydrin in the same way as the other amino acids because their alpha amino group is part of a five membered ring. Click to see full answer.
What is the color of ninhydrin and proline?
Now, the ammonia goes on to react with another ninhydrin molecule to form diketohydrin (which is also known as Ruhemann’s complex). This complex is responsible for the deep blue colour. When the analyte contains Imino-acids like proline, a yellow coloured complex is formed. When asparagine is used, the colour of the resulting complex is brown.
What is ninhydrin test with example?
Apr 22, 2020 · Proline and hydroxy-proline do not react with ninhydrin in the same way as the other amino acids because their alpha amino group is part of a five membered ring. Similarly one may ask, why does ninhydrin react with all amino acids? Ninhydrin reacts with the α-amino group of primary amino acids producing 'Ruhemann's purple'.
What is the chemical reaction of ninhydrin?
By the reaction with primary amino acids, a typical blue-violet-colored compound (Ruhemann purple, Figure 6.1) is formed [2,3], and a yellow-colored compound by the reaction with proline and hydroxyproline. Normally, the amino acids are separated by the cation-exchange column, and the column effluent is mixed with ninhydrin reagent.
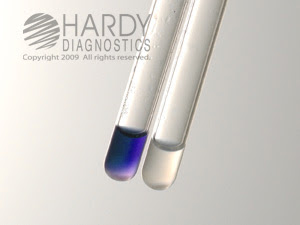
Why is proline not react with ninhydrin?
Ninhydrin is a strong oxidising agent when it reacts with alpha amino acids gives purple color which is known as ruhemanns purple complex, this is the result of the reaction of ninhydrin and amines present in the primary amino acids, prolin has a ring structure so nitrogen is not free to react with the ninhydrin as it ...
Is proline positive for ninhydrin test?
Ninhydrin Test Result Interpretation For ammonia, primary/secondary amines, and amino acids, deep purple colour is obtained. For hydroxyproline and proline, a yellow colour is obtained.
What color is proline with ninhydrin?
Proline gives a yellow colour because it is a secondary amine. Most amino acids are primary amines with the general structure H2NCHRCOOH . Except for proline and hydroxyproline, all the α-amino acids are oxidized by ninhydrin to give the same intensely colored purple anion.Jul 24, 2017
What amino acid does ninhydrin react with?
Ninhydrin is used in many bioanalytical techniques particularly for amino acid analysis method. Ninhydrin reacts with the α-amino group of primary amino acids producing 'Ruhemann's purple'. The chromophore formed is the same for all primary amino acids.
Does proline have a secondary amino group?
Proline contains a secondary amine group, called an imine, instead of a primary amine group. For this reason, proline is called an imino acid.
Is proline a structure?
The molecular formula of proline is C5H9NO2 and molecular mass being 115.13 g mol-1. The IUPAC name of proline is Pyrolidine-2-carboxylic acid; therefore, it is the secondary amino group known as imino group which belongs to a five-member ring in a molecule.
What colour would you expect the proline ninhydrin reaction to yield and why?
Imino acids, e.g. proline and hydroxyproline, react with ninhydrin to give a yellow color. At higher temperatures ("~100°C), the yellow compound (X) is trans- formed to the purple-red compound (XI) 1°.
Is proline an imino acid?
Proline. Proline contains a secondary amine group, called an imine, instead of a primary amine group. For this reason, proline is called an imino acid.
What colour is ninhydrin?
purple violet colorNinhydrin, a well-known nonselective color reagent for amino acids, is best used in neutral conditions and produces a purple violet color with all amino acids, except proline and hydroxyproline, because of the presence of secondary cyclic amine groups.
How does the structure of proline differ from those of other amino acids?
Proline has a unique structure compared to other amino acids because its R-group is bonded not only to a central carbon atom but to part of its amino group as well. Though our bodies synthesize proline, some people supplement this amino acid to improve their joint health and maintain youthfulness.
Why does ninhydrin react with amino acids?
First, ninhydrin is dehydrated and reacts with an amino acid, forming a Schiff base. Then, it undergoes decarboxylation, releasing a carbon dioxide. Finally, with its reaction with water, the bond with side chain (R group) then quickly departs from the imino intermediate, forming an aldehyde and diketohydrindamine.
Which amino acid does not give ninhydrin test?
Proline being a secondary amine give a yellow orange colour with ninhydrin whereas all other α− amino acids give a blue-purple colour with ninhydrin.
What reacts with ninhydrin?
Ninhydrin reacts with the α-amino group of primary amino acids producing 'Ruhemann's purple'. The chromophore formed is the same for all primary amino acids. The intensity of the colour formed depends on the number and chemical nature of the amino groups being analysed.
What is the reaction between ninhydrin and amino acids?
Furthermore, what is the reaction between ninhydrin and amino acid? In summary, ninhydrin, which is originally yellow, reacts with amino acid and turns deep purple. It is this purple color that is detected in this method. Ninhydrin will react with a free alpha-amino group, NH2-C-COOH. This group is present in all amino acids, proteins or peptides.
Why is ninhydrin used to detect fingerprints?
Ninhydrin is used to detect fingerprints because it reacts with amino acids from the proteins in skin cells transferred to the surface by the individual leaving the fingerprint. Click to see full answer.
Does nitrogen react with ninhydrin?
The nitrogen is the only portion of the purple anion that is derived from the amino acid. Proline and hydroxy-proline do not react with ninhydrin in the same way as the other amino acids because their alpha amino group is part of a five membered ring. Likewise, why does ninhydrin react with all amino acids?
What is the color of ninhydrin?
The dark purple coloration in ninhydrin-developed fingermarks may be modified by treatment with a metal salt solution. For example, treatment with a zinc (II) salt gives an orange color while that produced by a cadmium (II) salt is red.
What is ninhydrin used for?
Since its discovery by Ruhemann in 1910 [1], this colorimetric reaction has been widely used for the detection of amino acids, peptides, proteins, and amines. By the reaction with primary amino acids, ...
What is the name of the compound that is used to detect latent fingerprints?
The compound reacts with the amino acid (eccrine) component of the fingerprint deposit to give a dark purple product known as Ruhemann's purple (Figure 4 ).
What is the reaction of OPA?
OPA reacts with most of the amino acids (except cysteine, proline, and hydroxyproline) under alkaline conditions in the presence of reducing reagents, such as 2-mercaptoethanol, to generate a bright blue fluorescence.
Does ninhydrin react with amino acids?
However, not all amino acids react equally well with ninhydrin, and the detection limit is ∼50 pmol (∼6 ng) of amino acid derivative. The product of the reaction of ortho -phthaldialdehyde (OPA) with amino acids in the presence of thiol will fluoresce with excitation at 340 nm and emission at 450 nm.
Most recent answer
Sorry for my late response but I didn’t received a new from researchgate notification in my email. Maybe the alcohol may do a different reaction. In my case I dissolved the ninhydrin using acid, glacial acetic acid and phosphoric acid 85% (60/40 v/v).
All Answers (10)
The reaction of amino acids with ninhydrin is colorimetric. It is used to detect ammonia or primary and secondary amines and a deep blue or purple color known as Ruhemann's purple is produced.
What color is a proline?
The typical positive result is a blueish-purple color, as seen in the image below: (Image credit: pdecell) But, there is an amino acid that gives a yellow result. This amino acid is PROLINE. ( Image Credit) As you can see, proline has a ring structure (secondary amino acid). The blueish-purple result is usually associated with primary amino acids.
What is the purpose of ninhydrin test?
The Ninhydrin Test is used to test for the presence of amino acids (NOT proteins). Ninhydrin degrades amino acids into aldehydes, ammonia, and CO 2 (carbon dioxide) through a series of reactions; the net result is ninhydrin in a partially reduced form hydrindantin: Ninhydrin then condenses with ammonia and hydrindantin to produce ...
Is proline a primary amino acid?
As you can see, proline has a ring structure (secondary amino acid). The blueish-purple result is usually associated with primary amino acids. In these amino acids, the N is free to react with ninhydrin. However, in proline, the N is not available for reaction as it is locked in the ring structure. Therefore no ammonia is produced, so no blue color ...
