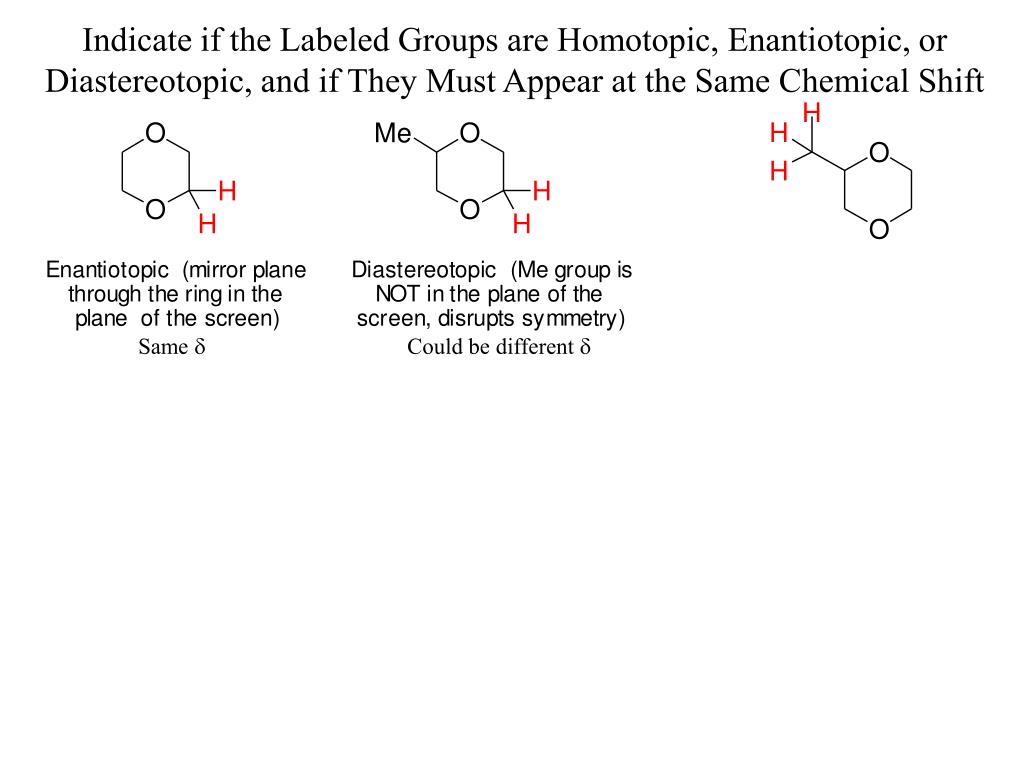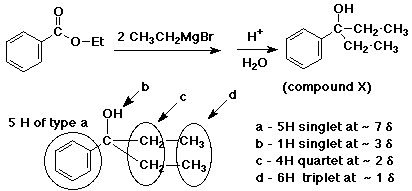
How much Oh does the Oh Proton show up in NMR?
I think the OH proton shows up at around 3.5-4 ppm. Some universities say that the OH proton does not show up, because in some instances it reacts with the solvent, so that there is a proton transfer occurring while the NMR is being taken.
What are the signals of-Oh and-NH groups in NMR?
The signals of -OH and -NH- groups in NMR appear to be a single peak. This is because the protons from these groups get exchanged with the proton of water or an acid.
Why do oh and NH peaks appear as single peaks in NMR?
The signals of -OH and -NH- groups in NMR appear to be a single peak. This is because the protons from these groups get exchanged with the proton of water or an acid. This exchange is reversible and so rapid, hence the peaks coincide and appear as one single peak.
Why do the peaks disappear in NMR spectroscopy?
The peaks that disappear are mainly the OH and NH peaks as a result of proton deuterium exchange. Also the OH and NH peaks usually appear broad and rarely split especially when the NMR was run on the sample at room temperature and the proton exchange between the OH and NH protons and that of the protic solvent appears very fast.
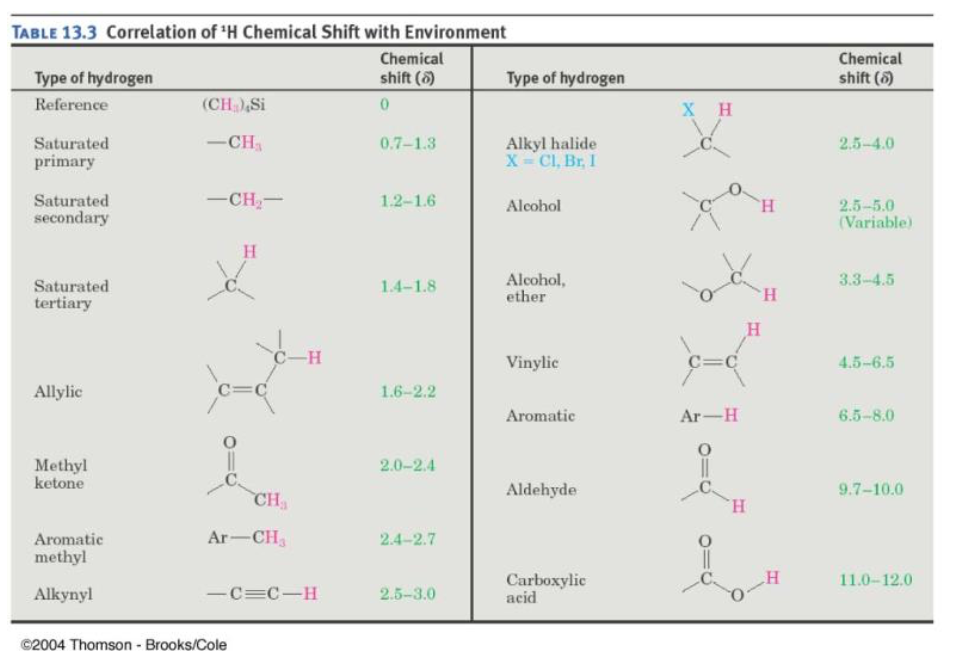
Where does OH show on NMR?
The 1H NMR chemical shifts for phenols are not particularly distinctive. However, one expects the −OH signal to be in the 4–7 ppm range, while the aromatic protons (see Section 15.7) are expected to be found at 7–8 ppm.
Is OH shielding or Deshielding?
Protons that are involved in hydrogen bonding (i.e.-OH or -NH) are usually observed over a wide range of chemical shifts. This is due to the deshielding that occurs in the hydrogen bond.
Why does OH have a broad peak NMR?
Generally in protic solvents the -OH groups appear at room temperature as broad signals due to fast, on the NMR time scale, exchange of the OH protons with protons of the solvents [20]. By decreasing the temperature, the proton exchange rate is reduced and relatively sharp –OH peaks are revealed.
What is shielded and Deshielded in H NMR?
These H atoms are referred to as being shielded. If the H atom is surrounded by elements that reduce the electron cloud, then, it would experience a higher magnetic field and would resonate at a higher radio frequency. This phenomenon is called de-shielding.
What causes Deshielding in NMR?
There are two major factors that cause different chemical shifts (a) deshielding due to reduced electron density (due electronegative atoms) and (b) anisotropy (due to π bonds). Coupling = Due to the proximity of "n" other equivalent H atoms, causes the signals to be split into (n+1) lines.
Does OH affect splitting in NMR?
Unless the alcohol is absolutely free of any water, the hydrogen on the -OH group and any hydrogens on the next door carbon don't interact to produce any splitting. The -OH peak is a singlet and you don't have to worry about its effect on the next door hydrogens.
Why do I have a carboxylic acid (- OH peak missing in an NMR spectrum?
You don't get the OH peak of carboxylic acids because besides being taken up by the moisture it may simply not be present as -OH, rather -O(Na) [or with some other metals] might have formed while workup or due to any reaction conditions.
What is shielding and Deshielding?
On Professor Hardinger's website, shielded is defined as “a nucleus whose chemical shift has been decreased due to addition of electron density, magnetic induction, or other effects.” What is Deshielding? Downfield The Nucleus feels stronger magnetic field. Deshielding is the opposite of shielding.
Is Deshielded upfield or downfield?
It is often convienient to describe the relative positions of the resonances in an NMR spectrum. For example, a peak at a chemical shift, δ, of 10 ppm is said to be downfield or deshielded with respect to a peak at 5 ppm, or if you prefer, the peak at 5 ppm is upfield or shielded with respect to the peak at 10 ppm.
How can you tell if a proton is shielded?
6:2011:49Shielding and Deshielding - H NMR Spectroscopy - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd which one is de-shielded which proton is in an electron-rich environment and which one is in anMoreAnd which one is de-shielded which proton is in an electron-rich environment and which one is in an electron poor environment. Now what we need to do is consider the effect of br on this molecule.
Which proton is most Deshielded?
phenolic protonIn this case, the phenolic proton is by far the most deshielded of all protons in the molecules shown; I'd expect a chemical shift of over 10ppm.
Popular Answers (1)
You may want to check out my publications "On Hydrogen Bonding and OH chemical Shifts" Parts I and II, and "On NH NMR Chemical Shifts", Parts I - Part IV; they contain detailed explanations, many examples from the literature, as well as many references. Full texts are free on Research Gate.
All Answers (24)
As you know the shielding and the deshielding process effect the position, appearance and disappearance of the protons. Also the nature of your molecule , solvent , hydrogen bonding ( inter and intra) and temperature all these factors may effect proton behavior .
Similar questions and discussions
How to interpret -NH and -OH peaks in proton NMR if DMSO is the solvent?
Which group of molecules give broad, and sometimes, deuterium-exchangeable signals?
Other groups that give broad, and sometimes, deuterium-exchangeable signals are the amines, amides, and thiols. And one more thing, which we will discuss in the signal splitting, is that the OH signal is not split by adjacent protons unless the sample is very well-dried.
Why do hydrogens of external alkynes resonate at lower frequency than vinylic hydrogens at appear at 2-3 pp
However, hydrogens of external alkynes resonate at lower frequency than vinylic hydrogens at appear at 2-3 ppm range. The reason is that, unlike alkenes , the induced magnetic field of the p electrons in the triple bond is opposite to the applied magnetic field.
Which aromatic compounds have inner hydrogens?
Interestingly, aromatic compounds with inner hydrogens such as, for example, porphyrins, [18]-annulene and the ones with hydrogens over the ring are shielded by the induced magnetic field and appear scientifically upfield:
What is the effect of electron withdrawing groups on the chemical shift?
The effect of electron-withdrawing groups on the chemical shift can be visualized by the image below: The stronger the electron-withdrawing group, the more deshielded the adjacent protons and higher their ppm value.
How many ppm does OH show up in a proton?
I think the OH proton shows up at around 3.5-4 ppm. Some universities say that the OH proton does not show up, because in some instances it reacts with the solvent, so that there is a proton transfer occurring while the NMR is being taken. I know, it's stupid and I wish it were more straightforward but for the MCAT I would assume that if you see an proton anywhere on any functional group, to assume that it will show up on the spectrum
Do OH bonds show up in NMR?
OH bonds do show up on NMR. I forgot what it was for alcohols, but carboxylic acids show up at about 12 ppm. H NMR will always show a peak if there is a hydrogen, no questions about it.
Can aromatic rings be merged?
All Answers (5) Yes it can be merged with aromatic ring and possible to go more downfield depends on aromatic rings (fused ring system/of any other electron withdrawing group attached to the same Ar-ring).
Is it possible to interpret a compound as an aromatic or aliphatic?
This interpret is dependent on of many conditions such as type structure for compound ( Aromatic or Aliphatic) , Hydrogen bonds (internal or external) , type of solvent and relaxation time . Yes, it is possible. It usually appear as a broader one.