Is pulmonary edema and pneumonia the same?
The major difference being that pneumonia is an infectious pathology while pulmonary edema is not usually caused by an infection. It is a marker for a more severe underlying systemic pathology like heart failure or volume overload states in the body. Pulmonary edema can also be a sequel of causes that fluid overload in the lung.
What is the prognosis for respiratory alkalosis?
The prognosis of respiratory alkalosis is variable and depends on the underlying cause and the severity of the underlying illness. Lewis et al hypothesized that respiratory alkalosis may interfere with vitamin D production, contributing to the development of fibromyalgia.
How to correct respiratory acidosis?
When the acidosis is acute and severe, a person needs emergency medical treatment to:
- restore healthy breathing
- restore the acid-base balance
- treat the cause of the respiratory failure
Is pnemonia a disease?
Pneumonia is a very serious disease that could cause life-threatening complications and result to adverse situations. It is a lung condition where lungs become affected in such a way that a person has breathlessness and other complications. It inflames the ...

Is there respiratory acidosis in pneumonia?
Respiratory acidosis may be acute or chronic (Tables 14.1 and14.2). Certain causes of chronic respiratory acidosis (e.g., chronic obstructive pulmonary disease [COPD]) can superimpose an element of acute respiratory acidosis during periods of decompensation (e.g., pneumonia, major surgery, heart failure).
Why does pneumonia cause respiratory alkalosis?
When you breathe faster, the lower carbon dioxide level in your blood can lead to respiratory alkalosis. Respiratory alkalosis is usually caused by over-breathing (called hyperventilation) that occurs when you breathe very deeply or rapidly.
Does pneumonia cause acid-base imbalance?
It has been concluded from the observations thus far that the acid-base condition of patients suffering from pneumonia is usu- ally within normal pH, CO, content and COz tension limits.
What causes respiratory acidosis and alkalosis?
Respiratory alkalosis occurs when you breathe too fast or too deep and carbon dioxide levels drop too low. This causes the pH of the blood to rise and become too alkaline. When the blood becomes too acidic, respiratory acidosis occurs.
What conditions cause respiratory alkalosis?
Respiratory alkalosis is a condition marked by a low level of carbon dioxide in the blood due to breathing excessively....Common causes include:Anxiety or panic.Fever.Overbreathing (hyperventilation)Pregnancy (this is normal)Pain.Tumor.Trauma.Severe anemia.More items...•
What conditions cause respiratory acidosis?
Causes of Chronic Respiratory AcidosisChronic obstructive pulmonary disease (COPD); a group of airflow and breathing diseases that include diseases like emphysema and bronchitis.Asthma.Diseases that happen in the lung tissue like pulmonary fibrosis.Muscular or nerve diseases.Obesity.Sleep apnea.More items...•
Can pneumonia cause metabolic acidosis?
All of our study children had both pneumonia and diarrhea. Acidosis in pneumonia is usually respiratory in origin [26] and on the other hand, diarrhea leads to metabolic acidosis [6]. Thus, the acidosis in our study population is likely to be mixed acidosis.
Which acid base imbalance can result from pneumonia hypoventilation or obstruction of airways?
Respiratory acidosis as a primary disorder is often caused by hypoventilation. This can be due to multiple causes including chronic obstructive pulmonary disease, opiate abuse/overdose, severe obesity, and brain injury.
Which of the following can cause respiratory alkalosis quizlet?
Anxiety with hyperventilation is the most common cause of respiratory alkalosis; therefore anxiety disorders increase the risk for this acid-base imbalance.
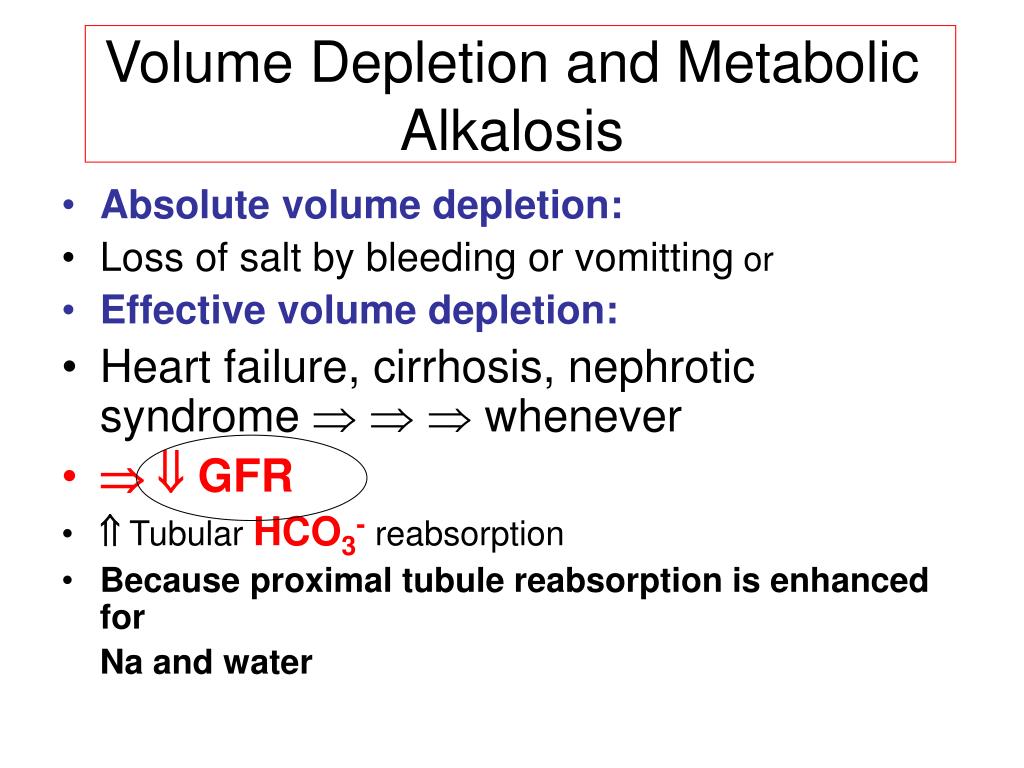
What leads to metabolic alkalosis?
Metabolic alkalosis is primary increase in bicarbonate (HCO3−) with or without compensatory increase in carbon dioxide partial pressure (Pco2); pH may be high or nearly normal. Common causes include prolonged vomiting, hypovolemia, diuretic use, and hypokalemia.
How can you distinguish between respiratory metabolic acidosis and alkalosis?
Metabolic acidosis: patients who are acidotic and have a HCO3– <22 (base excess <–2); Respiratory acidosis: patients who are acidotic with a PaCO2 >6; Metabolic alkalosis: patients who are alkalotic with a HCO3– >28 (base excess >+2); Respiratory alkalosis: patients who are alkalotic with a PaCO2 <4.7.
What causes respiratory and metabolic acidosis?
Respiratory acidosis develops when there is too much carbon dioxide (an acid) in the body. This type of acidosis is usually caused when the body is unable to remove enough carbon dioxide through breathing. Other names for respiratory acidosis are hypercapnic acidosis and carbon dioxide acidosis.
Why does pneumonia cause respiratory acidosis?
Inadequate Lung Tissue Ventilation and Perfusion When there is a mismatch between airflow (ventilation) and blood flow (perfusion), this leads to a condition called dead space ventilation. This loss of function can contribute to respiratory acidosis4 and may be due to: Pneumonia.
What is the pathophysiology of respiratory alkalosis?
Respiratory alkalosis is a pathology that is secondary to hyperventilation. Hyperventilation typically occurs in response to an insult such as hypoxia, metabolic acidosis, pain, anxiety, or increased metabolic demand. Respiratory alkalosis in itself is not life-threatening; however, the underlying etiology may be.
Why does pulmonary embolism cause respiratory alkalosis?
Thus, most patients with PE present with a lower than normal arterial PCO2 and respiratory alkalosis because of an increased total minute ventilation. Limited data suggest that the increased total minute ventilation occurs because of reflex stimulation of irritant and juxta capillary sensors in the lung.
How does fever cause respiratory alkalosis?
Key Points. Respiratory alkalosis involves an increase in respiratory rate and/or volume (hyperventilation). Hyperventilation occurs most often as a response to hypoxia, metabolic acidosis, increased metabolic demands (eg, fever), pain, or anxiety.
What is respiratory acidosis?
Respiratory acidosis is a medical emergency in which decreased ventilation (hypoventilation) increases the concentration of carbon dioxide in the blood and decreases the blood's pH (a condition generally called acidosis). Carbon dioxide is produced continuously as the body's cells respire, and this CO2 will accumulate rapidly if the lungs do not adequately expel it through alveolar ventilation. Alveolar hypoventilation thus leads to an increased PaCO2 (a condition called hypercapnia). The increase in PaCO2 in turn decreases the HCO3−/PaCO2 ratio and decreases pH. Terminology Acidosis refers to disorders that lower cell/tissue pH to < 7.35. Acidemia refers to an arterial pH < 7.36. [1] Types of respiratory acidosis Respiratory acidosis can be acute or chronic. In acute respiratory acidosis, the PaCO2 is elevated above the upper limit of the reference range (over 6.3 kPa or 45 mm Hg) with an accompanying acidemia (pH <7.36). In chronic respiratory acidosis, the PaCO2 is elevated above the upper limit of the reference range, with a normal blood pH (7.35 to 7.45) or near-normal pH secondary to renal compensation and an elevated serum bicarbonate (HCO3− >30 mm Hg). Causes Acute Acute respiratory acidosis occurs when an abrupt failure of ventilation occurs. This failure in ventilation may be caused by depression of the central respiratory center by cerebral disease or drugs, inability to ventilate adequately due to neuromuscular disease (e.g., myasthenia gravis, amyotrophic lateral sclerosis, Guillain–Barré syndrome, muscular dystrophy), or airway obstruction related to asthma or chronic obstructive pulmonary disease (COPD) exacerbation. Chronic Chronic respiratory acidosis may be secondary to many disorders, including COPD. Hypoventilation Continue reading >>
What is the mechanism responsible for lowering arterial pCO2 in all cases of respiratory alkalosis?
Hyperventilation is the mechanism in ALL cases Hyperventilation (ie increased alveolar ventilation) is the mechanism responsible for the lowered arterial pCO2 in ALL cases of respiratory alkalosis. This low arterial pCO2 will be sensed by the central and peripheral chemoreceptors and the hyperventilation will be inhibited unless the patients ventilation is controlled. 1. Central Causes (direct action via respiratory centre) Other 'supra-tentorial' causes (pain, fear, stress, voluntary) Various drugs (eg analeptics, propanidid, salicylate intoxication) Various endogenous compounds (eg progesterone during pregnancy, cytokines during sepsis, toxins in patients with chronic liver disease) 2. Hypoxaemia (act via peripheral chemoreceptors) Respiratory stimulation via peripheral chemoreceptors 3. Pulmonary Causes (act via intrapulmonary receptors) 4. Iatrogenic (act directly on ventilation) Can a decreased CO2 production cause respiratory alkalosis? Hyperventilation is the mechanism in all of the situations in the above list & indeed in all cases. Theoretically, a decreased carbon dioxide production could result in respiratory alkalosis if alveolar ventilation remained fixed. But this would not occur in a normal person because any drop in arterial pCO2 would reflexly cause a decreased ventilation (via chemoreceptor inhibitory input into the respiratory centre). About the only situation where maybe a decrease in CO2 production could be the mechanism of respiratory alkalosis would be in an intubated patient on fixed ventilation during Anaesthesia or in Intensive Care Unit and where the CO2 production was low due to hypothermia and decreased metabolic rate. However, even in such a circumstance, this mechanism is usually referred to as 'excessive controlled ventilation' (which it Continue reading >>
How does acidosis occur?
Acidosis is caused by an overproduction of acid in the blood or an excessive loss of bicarbonate from the blood (metabolic acidosis) or by a buildup of carbon dioxide in the blood that results from poor lung function or depressed breathing (respiratory acidosis). If an increase in acid overwhelms the body's acid-base control systems, the blood will become acidic. As blood pH drops (becomes more acidic), the parts of the brain that regulate breathing are stimulated to produce faster and deeper breathing (respiratory compensation). Breathing faster and deeper increases the amount of carbon dioxide exhaled. The kidneys also try to compensate by excreting more acid in the urine. However, both mechanisms can be overwhelmed if the body continues to produce too much acid, leading to severe acidosis and eventually heart problems and coma. The acidity or alkalinity of any solution, including blood, is indicated on the pH scale. Metabolic acidosis develops when the amount of acid in the body is increased through ingestion of a substance that is, or can be broken down (metabolized) to, an acid—such as wood alcohol (methanol), antifreeze (ethylene glycol), or large doses of aspirin (acetylsalicylic acid). Metabolic acidosis can also occur as a result of abnormal metabolism. The body produces excess acid in the advanced stages of shock and in poorly controlled type 1 diabetes mellitus (diabetic ketoacidosis). Even the production of normal amounts of acid may lead to acidosis when the kidneys are not functioning normally and are therefore not able to excrete sufficient amounts of acid in the urine. Major Causes of Metabolic Acidosis Diabetic ketoacidosis (buildup of ketoacids) Drugs and substances such as acetazolamide, alcohols, and aspirin Lactic acidosis (buildup of lactic acid Continue reading >>
What causes acid in the lungs?
Respiratory Acidosis Definition Respiratory acidosis is a condition in which a build-up of carbon dioxide in the blood produces a shift in the body's pH balance and causes the body's system to become more acidic. This condition is brought about by a problem either involving the lungs and respiratory system or signals from the brain that control breathing. Description Respiratory acidosis is an acid imbalance in the body caused by a problem related to breathing. In the lungs, oxygen from inhaled air is exchanged for carbon dioxide from the blood. This process takes place between the alveoli (tiny air pockets in the lungs) and the blood vessels that connect to them. When this exchange of oxygen for carbon dioxide is impaired, the excess carbon dioxide forms an acid in the blood. The condition can be acute with a sudden onset, or it can develop gradually as lung function deteriorates. Causes and symptoms Respiratory acidosis can be caused by diseases or conditions that affect the lungs themselves, such as emphysema, chronic bronchitis, asthma, or severe pneumonia. Blockage of the airway due to swelling, a foreign object, or vomit can induce respiratory acidosis. Drugs like anesthetics, sedatives, and narcotics can interfere with breathing by depressing the respiratory center in the brain. Head injuries or brain tumors can also interfere with signals sent by the brain to the lungs. Such neuromuscular diseases as Guillain-Barré syndrome or myasthenia gravis can impair the muscles around the lungs making it more difficult to breathe. Conditions that cause chronic metabolic alkalosis can also trigger respiratory acidosis. The most notable symptom will be slowed or difficult breathing. Headache, drowsiness, restlessness, tremor, and confusion may also occur. A rapid heart rate Continue reading >>
What causes respiratory acidosis?
Here is an article about respiratory acidosis Respiratory Acidosis. And here is one about respiratory alkalosis. 6.2 Respiratory Alkalosis - Causes. As you can see both are caused by an imbalance in the acid/base values in the body, but by different mechanisms in breathing patterns. Pneumonia can cause both disturbances in those patterns.
How does pneumonia affect the respiratory system?
Anyway as I said, the primary way that pneumonia affects respiratory function is in terms of impaired oxygenation, and the main way it does that is by causing alveoli to fill up with fluid. (Once the infection is gone, the fluid goes away too, by the way). But pneumonia also impairs oxygenation by making the work of breathing more difficult. This is because in terms of respiratory mechanics, the effect of having a bunch of alveoli filled with fluid is to make the lung behave as though it were "stiffer", i.e. it requires more inspiratory pressure to produce a given amount of inflation of the lung. (The term for this is reduced pulmonary "compliance"). This requires just plain more muscle energy for the muscles of breathing (diaphragm and chest wall muscles) to inflate the lungs, and the increased metabolic demand resulting from this additional effort required to breathe can really be bad news in a person who is quite ill. Being very sick is a hypermetabolic state, it takes a lot of energy to run a revved-up immune system, to mount a fever etc., and often these people can be rather dehydrated as well which makes things worse. Their decreased pulmonary function means that they may actually need to breathe faster and/or deeper, and their whole body system is meanwhile requiring more oxygen than usual in order to maintain this hypermetabolic state, and then the muscles of respiration which are having to work harder also need more oxygen, and it can be sort of a vicious circle with a real danger that the patient will tire out and decompensate and have trouble staying alive without the right treatments. So that's why a good assessment of respiratory function is important in people who come in quite ill with pneumonia :-).
How long does a respiratory infection last?
When treated, a typical upper respiratory tract infection clears spontaneously in one week. If untreated, however, it could last as long as seven days :)
What does pneumonia mean in the lung?
So then... pneumonia is a lung infection in which the inflammatory response of the lung tissue results in a build-up of fluid -- pus, basically -- that fills up some of the alveoli. What that means for those alveoli is that they can no longer participate in getting oxygen into your blood... they can't fill up with air because they're already filled up with fluid. So, these alveoli are basically "off-line", and overall this results in a decrease in the lungs' ability to oxygenate the blood.
What are some examples of restrictive lung disease?
Frequently, shortness of breath, cough, and respiratory failure will be observed. Some examples include pulmonary fibrosis, pneumonia, sarcoidosis and tuberculosis. The compliance of the lungs decreases, which makes the lungs stiffer.
Does pneumonia occur in acidosis?
Originally Answered: Pneumonia occurs in both acidosis and alkalosis. Why?
Is respiratory compromise a problem with pneumonia?
This is why the majority of people with pneumonia aren't actually experiencing any respiratory compromise at all, because even with part of a lung out of commission, their level of respiratory function is still more than adequate to supply the metabolic demands of the body at rest, and to maintain a blood oxygen saturation above about 94%. This is the situation for mild pneumonia in an individual who is healthy at baseline.
How to prevent acidosis?
The best way to prevent acidosis is to avoid causes of the disease. Choosing to live a smoke-free lifestyle may help. Smokers are at higher risk for chronic respiratory acidosis. Smoking is bad for lung function. It increases the risk of respiratory diseases and can have an adverse impact on overall quality of life.
What is the treatment for acute acidosis?
Treating acute acidosis usually means addressing the underlying cause. For example, your airway may need to be cleared. This must be done as soon as possible. Artificial ventilation may also be needed.
How does the kidneys work to remove acid from the blood?
The lungs remove acid by exhaling CO2 , and the kidneys excrete acids through the urine. The kidneys also regulate your blood’s concentration of bicarbonate (a base). Respiratory acidosis is usually caused by a lung disease or condition that affects normal breathing or impairs the lungs’ ability to remove CO2.
What is the condition where the lungs can't remove enough carbon dioxide?
Respiratory acidosis is a condition that occurs when the lungs can’t remove enough of the carbon dioxide (CO2) produced by the body. Excess CO2 causes the pH of blood and other bodily fluids to decrease, making them too acidic. Normally, the body is able to balance the ions that control acidity. This balance is measured on a pH scale from 0 to 14.
Why can't the lungs remove CO2?
However, sometimes the lungs can’t remove enough CO2. This may be due to a decrease in respiratory rate or decrease in air movement due to an underlying condition such as: asthma.
What is the pH of blood?
This balance is measured on a pH scale from 0 to 14. Acidosis occurs when the pH of the blood falls below 7.35 (normal blood pH is between 7.35 and 7.45). Respiratory acidosis is typically caused by an underlying disease or condition. This is also called respiratory failure or ventilatory failure. Normally, the lungs take in oxygen and exhale CO2.
What does high CO2 mean in blood?
A healthcare provider will take a sample of blood from your artery. High levels of CO2 can indicate acidosis.
What is respiratory acidosis?
Introduction. Respiratory acidosis is a state in which there is usually a failure of ventilation and an accumulation of carbon dioxide. The primary disturbance of elevated arterial PCO2 is the decreased ratio of arterial bicarbonate to arterial PCO2, which leads to a lowering of the pH. In the presence of alveolar hypoventilation, ...
Why is PCO2 elevated in respiratory acidosis?
In acute respiratory acidosis, there is a sudden elevation of PCO2 because of failure of ventilation.
What are the two features of alveolar hypoventilation?
In the presence of alveolar hypoventilation, 2 features commonly are seen are respiratory acidosis and hypercapnia. To compensate for the disturbance in the balance between carbon dioxide and bicarbonate (HCO3-), the kidneys begin to excrete more acid in the forms of hydrogen and ammonium and reabsorb more base in the form of bicarbonate.
What is the primary disturbance of elevated arterial PCO2?
The primary disturbance of elevated arterial PCO2 is the decreased ratio of arterial bicarbonate to arterial PCO2, which leads to a lowering of the pH.
What is the buffer system of carbon dioxide?
The buffer system created by carbon dioxide consists of the following three molecules in equilibrium: CO2, H2CO3-, and HCO3- . When H+ is high, HCO3- buffers the low pH. When OH- is high, H2CO3 buffers the high pH. In respiratory acidosis, the slight increase in bicarbonate serves as a buffer for the increase in H+ ions, which helps minimize the drop in pH. The increase in hydrogen ions inevitably causes a decrease in pH, which is the mechanism behind respiratory acidosis. [4][5]
How does carbon dioxide affect the body?
Carbon dioxide plays a remarkable role in the human body mainly through pH regulation of the blood. The pH is the primary stimulus to initiate ventilation. In its normal state, the body maintains CO2 in a well-controlled range from 38 to 42 mm Hg by balancing its production and elimination.
Which respiratory system controls alveolar ventilation?
The respiratory centers in the pons and medulla control alveolar ventilation. Chemoreceptors for PCO2, PO2, and pH regulate ventilation. Central chemoreceptors in the medulla are sensitive to changes in the pH level. A decreased pH level influences the mechanics of ventilation and maintains proper levels of carbon dioxide and oxygen. When ventilation is disrupted, arterial PCO2 increases and an acid-base disorder develop. Another pathophysiological mechanism may be due to ventilation/perfusion mismatch of dead space.
What is the compensatory change in PaCO2?
6: In compensation for metabolic alkalosis, the compensatory change in PaCO2 will be proportional to 0.6 times the SBE, i.e CO2 = 40 + (0.6 × SBE)
Does an acute change in PaCO2 change the standard base excess?
0: An acute change in PaCO2 will not change the Standard Base Excess.
Is respiratory acidosis a skill?
Respiratory acidosis and alkalosis are featured in virtually every paper, and being able to identify a respiratory acid-base disturbance is a vital skill for the CICM fellowship candidate. The SAQs will frequently require the application of the usual rules of compensation to reveal a hidden acid-base disorder, eg. "this patient has a low CO2 but it is not low enough ".
How does pneumonia affect the respiratory system?
Anyway as I said, the primary way that pneumonia affects respiratory function is in terms of impaired oxygenation, and the main way it does that is by causing alveoli to fill up with fluid. (Once the infection is gone, the fluid goes away too, by the way). But pneumonia also impairs oxygenation by making the work of breathing more difficult. This is because in terms of respiratory mechanics, the effect of having a bunch of alveoli filled with fluid is to make the lung behave as though it were "stiffer", i.e. it requires more inspiratory pressure to produce a given amount of inflation of the lung. (The term for this is reduced pulmonary "compliance"). This requires just plain more muscle energy for the muscles of breathing (diaphragm and chest wall muscles) to inflate the lungs, and the increased metabolic demand resulting from this additional effort required to breathe can really be bad news in a person who is quite ill. Being very sick is a hypermetabolic state, it takes a lot of energy to run a revved-up immune system, to mount a fever etc., and often these people can be rather dehydrated as well which makes things worse. Their decreased pulmonary function means that they may actually need to breathe faster and/or deeper, and their whole body system is meanwhile requiring more oxygen than usual in order to maintain this hypermetabolic state, and then the muscles of respiration which are having to work harder also need more oxygen, and it can be sort of a vicious circle with a real danger that the patient will tire out and decompensate and have trouble staying alive without the right treatments. So that's why a good assessment of respiratory function is important in people who come in quite ill with pneumonia :-).
What happens when you have pneumonia?
When you have a primary lung condition such as pneumonia, the amount of air that can be exchanged during a breath is reduced. The body compensates for the lower tidal volume by increasing the rate of breathing - essentially causing hyperventilation.
What does pneumonia mean in the lung?
So then... pneumonia is a lung infection in which the inflammatory response of the lung tissue results in a build-up of fluid -- pus, basically -- that fills up some of the alveoli. What that means for those alveoli is that they can no longer participate in getting oxygen into your blood... they can't fill up with air because they're already filled up with fluid. So, these alveoli are basically "off-line", and overall this results in a decrease in the lungs' ability to oxygenate the blood.
What happens when you hyperventilate?
When you hyperventilate, the amount of CO2 in your blood drops, and the pH of your blood increases. These are the chemical changes in the blood that characterize respiratory alk
Can pneumonia cause respiratory alkalosis?
Your observation should be: in pneumonia, one can have respiratory alkalosis, but also respiratory acidosis.
Is bronchopneumonia respiratory acidosis?
PS. To answer the question directly, in pneumonia, be it lobar or bronchopneumonia, breathing is impaired and respiration also, so it’s respiratory acidosis.
Is respiratory compromise a problem with pneumonia?
This is why the majority of people with pneumonia aren't actually experiencing any respiratory compromise at all, because even with part of a lung out of commission, their level of respiratory function is still more than adequate to supply the metabolic demands of the body at rest, and to maintain a blood oxygen saturation above about 94%. This is the situation for mild pneumonia in an individual who is healthy at baseline.
What is respiratory alkalosis?
Respiratory alkalosis occurs when high levels of carbon dioxide disrupt the blood’s acid-base balance. It often occurs in people who experience rapid, uncontrollable breathing (hyperventilation). Treatment includes supplemental oxygen and therapies to reduce the risk of hyperventilation.
Why is respiratory alkalosis important?
The condition is not life-threatening. Nor does it have lingering effects on your health. But it’s important to seek medical care for respiratory alkalosis because it’s often a sign of another medical condition. Some people need treatment with supplemental oxygen. Addressing what’s causing you to hyperventilate lowers your risk of future episodes.
Why does alkalosis occur?
Your body is continuously working to maintain the blood’s acid-base (alkali) balance. Alkalosis occurs when there’s too much alkali and not enough acid. Chemical changes in the acid-base balance can reflect changes in metabolism or breathing.
What causes rapid uncontrolled breathing?
People who experience intense bouts of stress, anxiety, panic or anger are at higher risk for respiratory alkalosis. These conditions can lead to rapid, uncontrolled breathing (hyperventilation).
What happens when you breathe faster?
Your body releases carbon dioxide when you exhale. When you breathe faster, the lower carbon dioxide level in your blood can lead to respiratory alkalosis.
Can a lung disease cause shortness of breath?
Any lung disease that leads to shortness of breath can also cause respiratory alkalosis (such as pulmonary embolism and asthma).