
The solubility of borax increases as temperature increases. As the solution cooled down, the borax begins to solidify and precipitates out, lowering the solubility at lower temperatures. Additionally, less of the HCl solution was required in order to bring the analyte to its endpoint.
What is the solubility product constant equation for borax and water?
Na2B4O7 * 10 H2O → 2 Na+ + B4O5(OH)42+ + 8H2O what is the solubility product constant equation for the reaction between borax and water? Ksp = [Na]^2[B4O5(OH)4] rewrite the Ksp formula from above in terms of [B4O5(OH)4]. Ksp = [Na]^2[B4O5(OH)4]
What is the enthalpy of dissolution of borax in water?
The literature values for enthalpy and entropy of the dissolution of borax in water are 110 kJ/mol and 380 J/mol-K, respectively. Determine the percent error in your experimentally determined values. Experimentally determined values: ΔHº = 153.06 kJ/mol and ΔSº = 471.4 J/mol*K
How does solubility affect the disorder of a system?
b. endothermic; because solubility (Ksp) increases as heat increases, heat can be considered a "reactant", therefore being consumed in the reaction c. increases solids are less disordered than liquids, which are less disordered than gases; because the reaction is solid --> liquid, disorder increases, so the disorder of the system (∆Ssys) increases
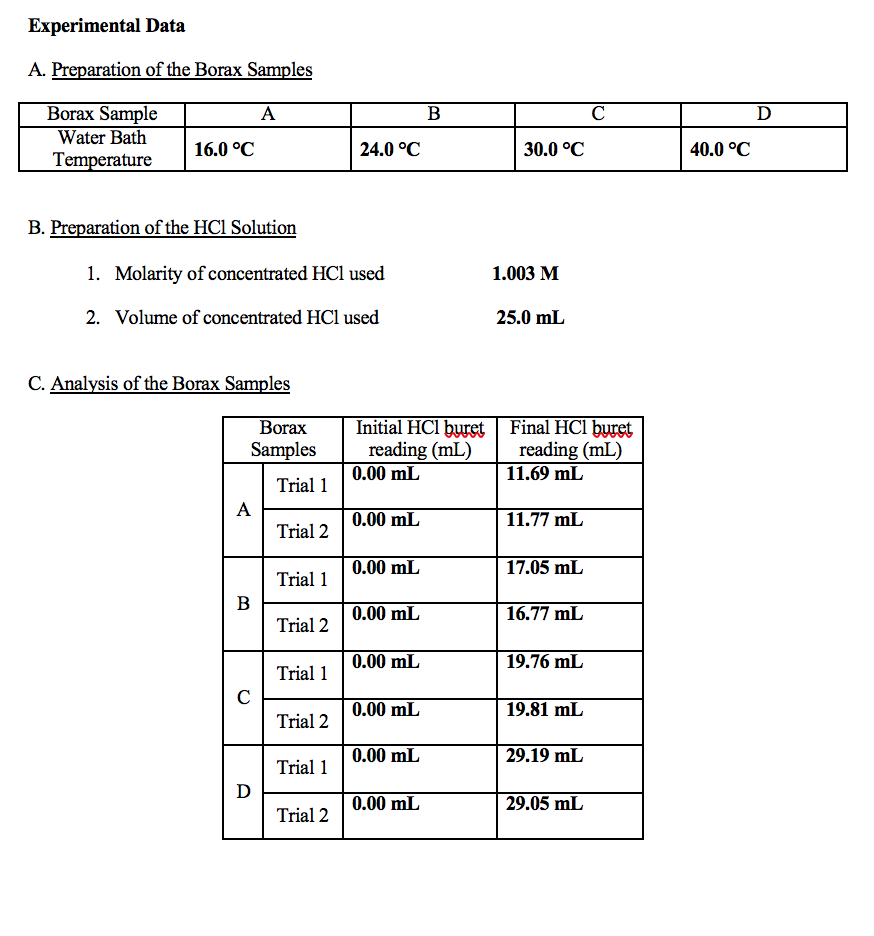
What is the trend in solubility of borax in water as the temperature increases?
When the temperature of the borax solution increases, then the dissolution of solid borax increases. The formation of ions increases the solubility of borax.
Do you expect the solubility of borax to increase or decrease as temperature increases quizlet?
- The solubility of borax in water increases as temperature increases. - More borax is dissolved at higher temperatures, indicating heat is a reactant.
Is the solubility of borax endothermic or exothermic?
endothermicThe dissolution (enthalpy or heat of solution) for borax (sodium tetraborate) is an endothermic process.
At what temperature does borax dissolve?
It is odorless, and has an unpleasant taste. Borax melts at 743 °C and boils at 1,575 °C. It has a density of 1.73 g/cm3. Borax is poorly soluble in cold water, but it's solubility increases with temperature.
What happens when borax is heated?
(a) When heated, borax undergoes various transitions. It first loses water molecules and swells. Then, it turns into a transparent liquid, solidifying to form a glass-like material called borax bead.
Does raising temperature increase solubility?
Increasing the temperature will therefore increase the solubility of the solute. An example of a solute whose solubility increases with greater temperature is ammonium nitrate, which can be used in first-aid cold packs. Ammonium nitrate dissolving in solution is an endothermic reaction.
Is borax soluble in hot water?
Borax slightly soluble in cold water, very soluble in hot water and insoluble in acids.
Is the dissolution of borax in water a temperature dependent?
Answer and Explanation: The dissolution of borax in water is an endothermic reaction so it is a temperature-dependent reaction. The reaction is non-spontaneous as heat is required as a reactant for borax to dissolve in water.
Does borax dissolve in cold water?
Borax and Hot Water Woes Some delicate fabrics, whether white or colored, are labeled as cold-wash only. However, borax readily dissolves only in warm or hot water.
Does temperature affect the formation of borax crystals?
The solubility of most solids increases with temperature. In other words, more Borax may be dissolved in hot water than cold water. So if a hot, saturated mixture is cooled, there's more Borax than can be contained by the colder water, and so Borax may fall out of the mixture, forming crystals.
How can you make borax dissolve faster?
By mixing the borax into hot water, instead of room temperature or cold water, the borax can stay suspended much longer. Very hot water can hold much more dissolved borax than cold water. Hot water molecules are moving very fast and are spread way out which makes space available for more borax to dissolve into it.
What is the solubility of borax?
BoraxNamesSolubility in water31.7 g/LMagnetic susceptibility (χ)−85.0·10−6 cm3/mol (anhydrous)Refractive index (nD)n1=1.447, n2=1.469, n3=1.472 (decahydrate)Structure48 more rows
Does solubility increases with decreasing temperature?
The solubility of most solid or liquid solutes increases with increasing temperature. The components of a mixture can often be separated using fractional crystallization, which separates compounds according to their solubilities. The solubility of a gas decreases with increasing temperature.
When solubility decreases does temperature increase?
Because, when temperature is increased, more energy is given to the system, which is used by the gas molecules to overcome the solvent-gas interactions and break free to move into the gaseous state. So, solubility of a gas in liquid decreases with an increase in temperature.
In which process solubility decreases with increase in temperature?
exothermic processIn an exothermic process, the solubility of a solute decreases with increase in temperature.
How does temperature affect solubility quizlet?
How does temperature affect solubility? increasing the temperature, increases the solubility for most solids. Increasing the temperature, decreases the solubility for gases.