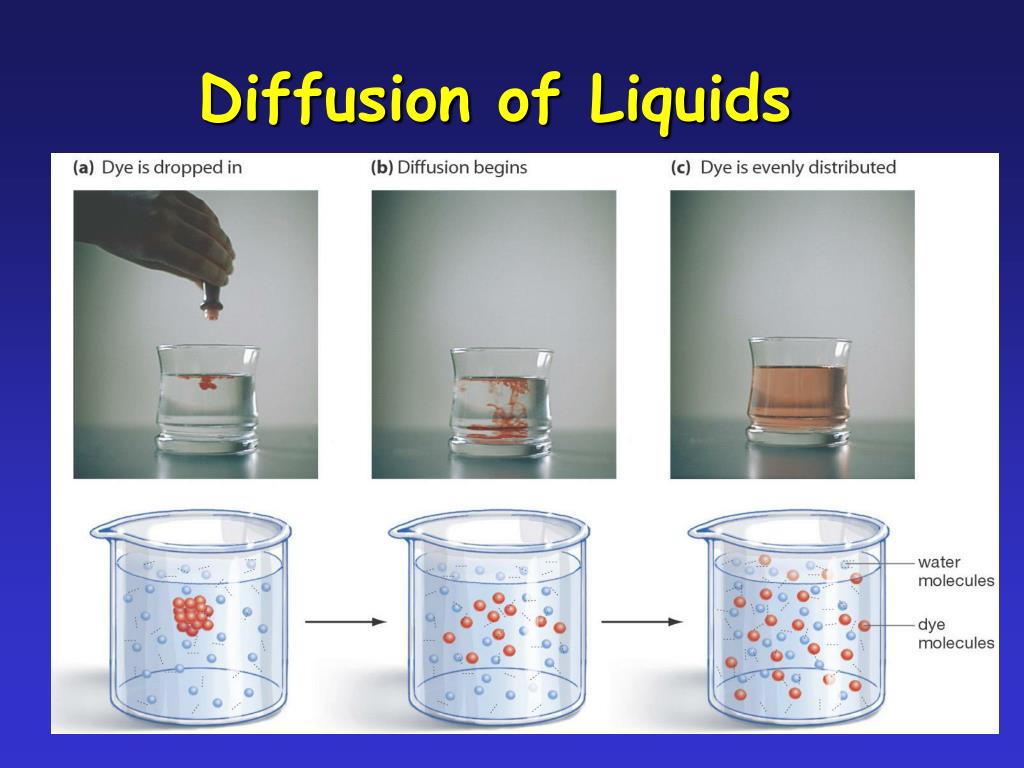
The dilution of water by solute resulting in lower concentration of water on the higher solute concentration side of the membrane and therefore a diffusion of water along the high to low concentration gradient occurs. .
What happens when the solute concentration of a substance changes?
The answer is: Diffusion moves from high solute concentration to low solute concentration. Molecules move from high concentration to low, just as surely as rocks roll downhill. Entropy goes up and it would require energy to reverse the process.
What happens to diffusion when the solute concentration decreases?
The answer is: Diffusion moves from high solute concentration to low solute concentration. Molecules move from high concentration to low, just as surely as rocks roll downhill. Entropy goes up and it would require energy to reverse the process. You need to distinguish between the diffusion of a solute and the diffusion of the solvent.
What is the effect of dilution on the concentration of water?
The dilution of water by solute resulting in lower concentration of water on the higher solute concentration side of the membrane and therefore a diffusion of water along the high to low concentration gradient occurs. .
How do molecules move from high concentration to low concentration?
Molecules move from high concentration to low, just as surely as rocks roll downhill. Entropy goes up and it would require energy to reverse the process. You need to distinguish between the diffusion of a solute and the diffusion of the solvent.
What happens to the concentration of solute in the right arm of the U tube?
As osmosis begins, the concentration of solute on the right arm concentrates, its density increases and simultaneously the pressure in the right arm increases which pushes the solvent into the left arm. While there are many theories, there is still no clear view why osmosis ...
How does a solvent move across the membrane?
The obvious question that arises is how the solvent separated by a membrane which is permeable to solvent moves across the membrane from a region of lower osmotic pressure to higher osmotic pressure overcoming the hydrostatic pressure. A movement of solvent from higher concentration to lower concentration implies that water moves ...
How to find osmotic pressure?
The best known expression for Osmotic pressure is below Π = iMRT where Π is the osmotic pressure in atm i = Van 't Hoff factor of the solute M = molar concentration in mol/L R = universal gas constant = 0.08206 L·atm/mol·K T = absolute temperature in K Osmotic pressure depends on [1] Van't Hoff factor [2]molar concentration of solute. Van’t Hoff’s factor, expresses the extent of association or dissociation of solutes in solution.It is the number of particles a solute dissociates in water. Example: for sucrose it is 1 and for NaCl it is 2
What is the pressure applied to stop the flow of water across the membrane?
It can also be restated that osmosis involves movement of water across a semipermeable membrane separating two solutions of different concentrations from a region of lower solute concentration to higher solute concentration. Osmotic pressure is the external pressure to be applied to stop flow of water across the membrane.
What is the process of separating two solutions of different concentrations?
Osmosis is a selective movement of solvent from a solution through a semipermeable membrane separating two solutions of different concentrations. Solvent moves from a region of higher concentration to a region of lower concentration. It can also be restated that osmosis involves movement of water across a semipermeable membrane separating two ...
Why is water more concentrated in osmosis?
Very simple explanation for osmosis is the concentration of water explanation - water in pure water is simply more concentrated than water in solutions because the solute has to take up some room in the solution. The dilution of water by solute resulting in lower concentration of water on the higher solute concentration side ...
What would happen if the bound water explanation were true?
If the bound water explanation were true, we would expect that a greater mass of hydrophilic solute would bind more water. Also, when predicting osmosis, we would have to carefully consider how hydrophilic the solute is (that is, how many water molecules it binds per molecule).
What happens when a solute moves from a low to high concentration?
solute moves from a low to high concentration, ATP is usually used, energy is released causes proteins to change conformation
How does a solute move?
solute will move from a high to low concentration (of solute), down its concentration gradient, no transport protein is used, no energy input needed. Only substances that are soluble in the lipid bilayer can pass through the membrane by simple diffusion.
What is the purpose of the ion gradient in a protein?
2. one protein uses the ion gradient as a source of energy to transport substance across the membrane-- against the concentration gradient
What happens to water when it is diffusion?
the diffusion of water, water moves from high to low concentration of water, no energy input needed
What does "water out" mean in biology?
water out < water in -- so water moves out of the cell and it shrivels up
Does solute move down the concentration gradient?
solute moves down the concentration gradient, no energy needed aka simple and facilitated diffusion
