Based on the results from the experiment, the temperature of water does affect the time it takes for sugar to dissolve, the line of best fit also shows the same results. The results I got showed that the warmer the water, the faster sugar dissolves.
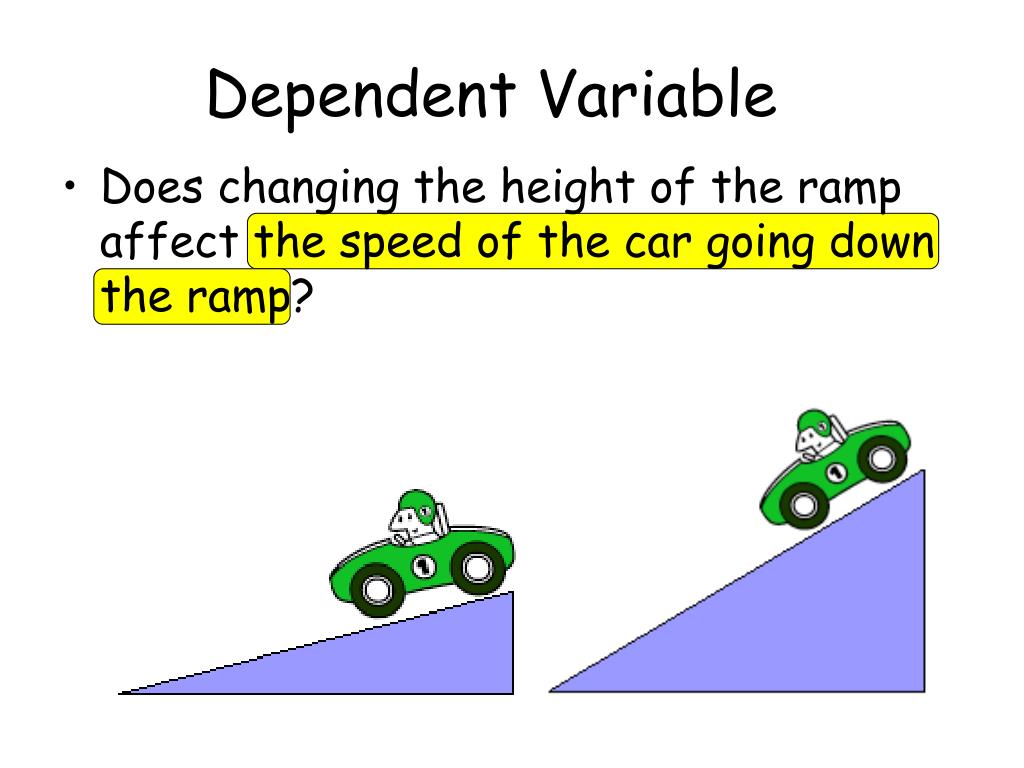
How does temperature affect the rate at which sugar cubes dissolve?
I think that the warmer the water is, the faster the sugar cube will dissolve in water. According to the Particle Theory, the warmer the water is, the more space there will be between the water particles because they move quicker. Below are the apparatus for the equipment used. 1.
How long does it take for sugar to dissolve in water?
It did dissolve entirely as a result of vigorous stirring action with water temperature measuring 35 degree Fahrenheit, but at a rate of two minutes and thirty one seconds for white sugar crystals and one minute and five seconds for the brown sugar crystals.
How does temperature affect the rate of dissolving?
Temperature as discussed is one of the factors that affect the rate of dissolving. The hot water measured 146 degrees Fahrenheit and was consistent with the hypothesis that the sugar will dissolve faster. Temperature affected the rate at which the white sugar cube dissolved slower than the brown sugar crystal and took about 37 seconds to dissolve.
What are the possible errors in the sugar dissolving experiment?
Evaluation Possible errors in this experiment are 1. Not waiting until the sugar is fully dissolved 2. The temperature of the water returning back to room temperature 3.
How does nitrogen work in coffee?
How many participants in the taste ability experiment?
How are macromolecule polymers assembled from monomers?
About this website
Does temperature affect the rate of dissolving the cube of sugar?
The reason why sugar dissolves at a faster rate in hot water has to do with increased molecular motion. The added energy in the hot water causes water molecules to move faster and sucrose molecules to vibrate faster.
How does temperature affect the rate of dissolving?
Temperature. Heating up a solvent gives the molecules more kinetic energy. The increased rapid motion means that the solvent molecules collide with the solute with greater frequency, and that the collisions occur with more force. Both factors increase the rate at which the solute dissolves.
How does temperature affect dissolving sugar?
Sugar dissolves faster in hot water than it does in cold water because hot water has more energy than cold water. When water is heated, the molecules gain energy and, thus, move faster. As they move faster, they come into contact with the sugar more often, causing it to dissolve faster.
Which factors affect the rate of dissolving of sugar?
The rate of dissolving is influenced by several factors, including stirring, temperature of solvent, and size of solute particles. When you add sugar to iced tea and then stir the tea, the sugar will dissolve faster.
Why does temperature affect the rate of solubility?
The Effect of Temperature on Solubility As the temperature increases, the particles of the solid move faster, which increases the chances that they will interact with more of the solvent particles. This results in increasing the rate at which a solution occurs.
How does temperature affect the rate of dissolution of salt in water?
Yes, salt and other ionic compounds like it will dissolve faster the hotter the water it is dissolved in. This is because hot temperatures make atoms move quicker and the quicker they move, the easier they come apart!
What are the 4 factors that affect the rate of dissolving?
UPLOAD PHOTO AND GET THE ANSWER NOW! Solution : The factors affecting rate of dissolving are
(i) Temperature of solvent, (ii) Size of solute particles, (iii) Stirring of the solution. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
How does the temperature of water affect the dissolving rate of salt?
Most solids, including sugar and salt, become more soluble with increasing temperature. This is because heat increases molecular movement, causing more collisions between the water molecules and the solid.
How Does Water Affect the Time It Takes for Sugar to Dissolve
When this happens the water molecules web together and the water becomes ice. The reason the salt and sugar lowers the freezing point and the water takes longer to freeze is because the salt or sugar molecules interfere with the attraction between the water molecules and therefore interfere with the webbing of the water molecules which prevents the water from turning into ice, when the water ...
How Does Water Affect the Time It Takes for Sugar to Dissolve
Procedure: First a 400mL beaker was filled 2/3 full with ice. Next the time and temperature were recorded every minute for 18 minutes. All the data was recorded in a data table.
How much time does it take for sugar to dissolve in water?
Answer (1 of 6): Time will depend upon many things. Amount of Sugar: More sugar - more time Amount of water: More water- less time Temperature of water: higher the temperature- less time Whether the contents are bring stirred. : With stirring- less time Crystal size of sugar. Fine sugars - l...
At what temperature does sugar dissolve? - Quora
Answer (1 of 7): Dissolve in what? I'll assume you mean water. C'mon, have you never had iced tea?. Wasn't it sweet?. Since there was ice in it, if stirred, the temperature couldn't be far from 0 C or 32 F. Sure, the solubility is lower the temperature of the water, but there is no temperature a...
Why does sugar dissolve in water - WhyCenter.com
Sugar has the propensity to dissolve readily in water. When sugar is poured in water, the sugar molecules split as the water molecules pull them away from each other, binding them to sugar molecules.
How does the temperature of water affect how fast sugar ... - StudyMode
We started setting up our experiment by measuring different molar solutions (0M, 0.25M, 0.5M, 0.75M and 1M) of sugar and putting them into separate bottles, along with one bag (12g) of yeast and 300ml of water.
1. Introduction
The purpose of my project is to demonstrate how water temperature affects the dissolving of sugar in liquid. Everything in our universe is made up of particles which are in constant motion. In a solid state particles move the slowest while in a liquid state particles move the fastest.
The Essay on Conductivity Of Streams Water Area Temperature
The measure of the capacity of water to allow an electrical current is called conductivity. Conductivity in streams is affected by many factors such as the elements or compounds found in the water, the temperature of the water, the geology of the area, and the pollution in the water. Each of these factors affect the conductiity in some way.
The Essay on How Does The Temperature Of Water Affect How Fast Sugar Can Dissolve?
Predict We will do three different tests, dissolving the water in boiling, warm and cool water. The sugar in the boiling water will dissolve the fastest. The sugar added to the cool water will dissolve the slowest.
5. Discussion
Temperature as discussed is one of the factors that affect the rate of dissolving. The hot water measured 146 degrees Fahrenheit and was consistent with the hypothesis that the sugar will dissolve faster. Temperature affected the rate at which the white sugar cube dissolved slower than the brown sugar crystal and took about 37 seconds to dissolve.
The Essay on Experimental Tube Respiration Rate Temperature
Introduction: Respiration commonly known as the inhalation and exhaling or breathing has a more little known definition. This is the definition that involves the cellular level of eukaryotic cells. Cellular respiration may best be described by the following equation: C 6 h 1206+602-6 CO 2+6 H 20+36 ATP.
6. Conclusion
Looking back on the results obtained from the data table, and how the different water temperature affected the rate at which the sugar dissolved, it is possible to draw several conclusions. As stated in my hypothesis, the increase in the temperature of water will affect the rate of dissolving by speeding up the process.
Request Removal
Note: The essay example you see on this page is a free essay, available to anyone. You are welcome to use this sample for your research! However, we strongly do not recommend using any direct quotes from this research paper for credit - you will most probably be caught for copying/pasting off the Internet.
How does nitrogen work in coffee?
Infusing the nitrogen into the coffee can take different forms. This can be done either through pressurization or through the use of a device known as a Nitrogenator. The Nitrogenator works in a similar manner to how water gets carbonated. Various methods can be utilized in brewing Nitro coffee. The coffee has to be held at a pressure that is at least 3 bars (40 psi). This maintains
How many participants in the taste ability experiment?
taste ability, psychology students conducted an experiment on 595 non diabetic participants. In the experiment, participants were asked to taste 8 different water-sugar solutions with different sugar strength, then were asked to state whether the solution had sugar in it or not. As hypothesised, female participants were more able to detect the sugar in the solution than male participants, also, non-smokers were able to get a higher hit rate than smokers. These findings suggest that the sense of taste
How are macromolecule polymers assembled from monomers?
Carbohydrates How are macromolecule polymers assembled from monomers? How are they broken down? To assemble a macromolecule polymer from monomers, the monomers must bond. This is a process known as a dehydration reaction, in which a water molecule is lost to form the bond. When this process occurs, each of the two bonded monomers provides part of the water molecule that was lost in the dehydration reaction: one contributes a hydroxyl group and the other a hydrogen. Dehydration reaction can take place over
How does nitrogen work in coffee?
Infusing the nitrogen into the coffee can take different forms. This can be done either through pressurization or through the use of a device known as a Nitrogenator. The Nitrogenator works in a similar manner to how water gets carbonated. Various methods can be utilized in brewing Nitro coffee. The coffee has to be held at a pressure that is at least 3 bars (40 psi). This maintains
How many participants in the taste ability experiment?
taste ability, psychology students conducted an experiment on 595 non diabetic participants. In the experiment, participants were asked to taste 8 different water-sugar solutions with different sugar strength, then were asked to state whether the solution had sugar in it or not. As hypothesised, female participants were more able to detect the sugar in the solution than male participants, also, non-smokers were able to get a higher hit rate than smokers. These findings suggest that the sense of taste
How are macromolecule polymers assembled from monomers?
Carbohydrates How are macromolecule polymers assembled from monomers? How are they broken down? To assemble a macromolecule polymer from monomers, the monomers must bond. This is a process known as a dehydration reaction, in which a water molecule is lost to form the bond. When this process occurs, each of the two bonded monomers provides part of the water molecule that was lost in the dehydration reaction: one contributes a hydroxyl group and the other a hydrogen. Dehydration reaction can take place over