
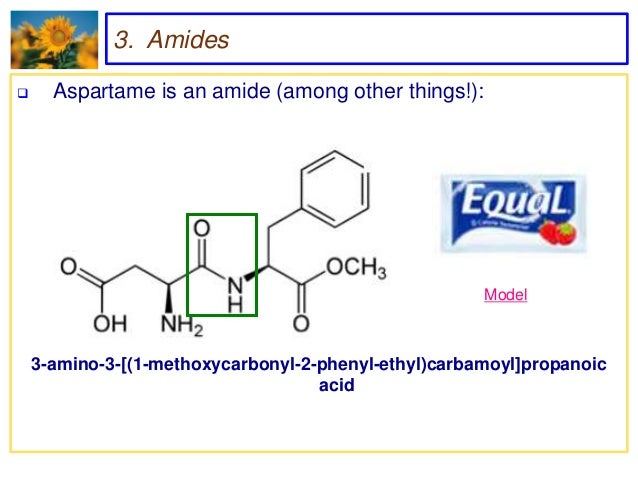
Nomenclature of Amides
- Primary amides. Primary amides are named by changing the name of the acid by dropping the -oic acid or -ic acid endings and adding -amide.
- Secondary Amides. Secondary amides are named by using an upper case N to designate that the alkyl group is on the nitrogen atom.
- Tertiary Amides. Try to draw a structure for N,N-dimethylformamide. ...
What does the name amides mean?
Amide, any member of either of two classes of nitrogen-containing compounds related to ammonia and amines. The covalent amides are neutral or very weakly acidic substances formed by replacement of the hydroxyl group (OH) of an acid by an amino group (NR 2, in which R may represent a hydrogen atom or an organic combining group such as methyl, CH 3).The carboxamides (R′CONR 2), which are ...
How are amides named?
Nomenclature of Amides
- Primary amides. Primary amides are named by changing the name of the acid by dropping the -oic acid or -ic acid endings and adding -amide.
- Secondary Amides. Secondary amides are named by using an upper case N to designate that the alkyl group is on the nitrogen atom.
- Tertiary Amides. Try to draw a structure for N,N-dimethylformamide.
How to name an amide?
Amides are amine derivatives of carboxylic acids. The root name is based on the longest chain including the carbonyl group of the amide group. Since the amide group is at the end of the chain, the C=O carbon must be C1. The amide suffix is appended after the hydrocarbon suffix minus the "e" : e.g. -ane + -amide = -anamide etc.
What does the name amide mean?
Summary
- Amides are functional group where carbonyl group attached to a amine group.
- The electrons of three p orbitals on three atoms oxygen, carbon and nitrogen are are on the same plane and delocalized.
- Normally the name starts with the name of long chain aliphatic and ends with -amide.

How do you name amides and amines?
Amines are named by naming the alkyl groups attached to the nitrogen atom, followed by the suffix -amine. Most amides are solids at room temperature; the boiling points of amides are much higher than those of alcohols of similar molar mass. Amides of five or fewer carbon atoms are soluble in water.
How do you name and draw an amide?
8:2710:14Examples for naming and drawing amides - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe parent name also always includes in that fork in that chain of carbons the carbonyl carbon andMoreThe parent name also always includes in that fork in that chain of carbons the carbonyl carbon and the carbonyl carbon always gets carbon number one there's carbon two carbon three.
How do you name amide bonds?
1:5511:25Naming Amides - IUPAC Nomenclature - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe're gonna have a methyl group attached to the nitrogen atom. So feel free to pause the video ifMoreWe're gonna have a methyl group attached to the nitrogen atom. So feel free to pause the video if you want to try naming this molecule so first what type of am I do we have is this a primary amide a
How do you name an amine?
Simple 1°, 2°, and 3° amines: common (trivial) names are obtained by alphabetically arranging the names of the alkyl substituents on the nitrogen and adding the suffix -amine (e.g., ethylmethylamine). Amines in the IUPAC system: the “e” ending of the alkane name for the longest chain is replaced with –amine.
How do you identify amides?
Key TakeawaysAmides have a general structure in which a nitrogen atom is bonded to a carbonyl carbon atom.The functional group for an amide is as follows:In names for amides, the -ic acid of the common name or the -oic ending of the IUPAC for the corresponding carboxylic acid is replaced by -amide.
What does N mean when naming amines?
nitrogen substituentsThe additional nitrogen substituents in 2º and 3º-amines are designated by the prefix N- before the group name.
How do you name an amine in organic chemistry?
Amines (R-NH2) are named for the attached alkane chain with the suffix “-amine” (e.g. CH3NH2 methanamine). If necessary, the bonding position is infixed: CH3CH2CH2NH2 propan-1-amine, CH3CHNH2CH3 propan-2-amine. The prefix form is “amino-“.
How do you name a nh2 group?
2:4518:06Naming Amines - IUPAC Nomenclature & Common NamesYouTubeStart of suggested clipEnd of suggested clipSo because we have two methyl groups attached to it. This is gonna be called dimethyl amine now whatMoreSo because we have two methyl groups attached to it. This is gonna be called dimethyl amine now what about this molecule n CH 2 CH 3 times 3 go ahead and name that molecule.
What is the suffix of amide?
The amide suffix is appended after the hydrocarbon suffix minus the "e" : e.g. -ane + -amide = -anamide etc. If the amide nitrogen is substituted, the these substituents are given N- as the locant.
What are amides in nature?
Amides are pervasive in nature and technology. Proteins and important plastics like Nylons, Aramid, Twaron, and Kevlar are polymers whose units are connected by amide groups ( polyamides ); these linkages are easily formed, confer structural rigidity, and resist hydrolysis.
What is the formula for amide?
In organic chemistry, an amide ( / ˈæmaɪd / or / ˈæmɪd / or / ˈeɪmaɪd / ( listen), also known as an organic amide or a carboxamide, is a compound with the general formula RC (=O)NR′R″, where R, R', and R″ represent organic groups or hydrogen atoms.
What is the name of the amide derived from acetic acid?
In the usual nomenclature, one adds the term "amide" to the stem of the parent acid's name. For instance, the amide derived from acetic acid is named acetamide (CH 3 CONH 2 ). IUPAC recommends ethanamide, but this and related formal names are rarely encountered.
What is the name of the amide formed from dimethylamine and acetic acid?
Thus, the amide formed from dimethylamine and acetic acid is N, N -dimethylacetamide (CH 3 CONMe 2, where Me = CH 3 ). Usually even this name is simplified to dimethylacetamide.
How much does acetamide contribute to the structure?
It is estimated that for acetamide, structure A makes a 62% contribution to the structure, while structure B makes a 28% contribution. (These figures do not sum to 100% because there are additional less-important resonance forms that are not depicted above).
What are the methods of peptide synthesis?
Conventional methods in peptide synthesis use coupling agents such as HATU, HOBt, or PyBOP.
Is an amide a weak base?
Basicity. Compared to amines, amides are very weak bases. While the conjugate acid of an amine has a p Ka of about 9.5, the conjugate acid of an amide has a p Ka around −0.5. Therefore, amides don't have as clearly noticeable acid–base properties in water.
What is an amide?
An amide is a functional group containing a carbonyl group linked to a nitrogen atom or any compound containing the amide functional group. Amides are derived from carboxylic acid and an amine.
What are some examples of amides?
Examples of Amides. Examples of amides include carboxamides, sulfonamides, and phosphoramides. Nylon is a polyamide. Several drugs are amides, including LCD, penicillin, and paracetamol.
What are amides used for?
Amides may be used to form resilient structural materials (e.g., nylon, Kevlar). Dimethylformamide is an important organic solvent. Plants produce amides for a variety of functions. Amides are found in many drugs.
What is the suffix for amide?
The - ic ending of the common name or the - oic ending of the International Union of Pure and Applied Chemistry (IUPAC) name of the carboxylic acid is replaced with the suffix - amide.
What is the structure of an amide?
Amides have a general structure in which a nitrogen atom is bonded to a carbonyl carbon atom. In names for amides, the - ic acid of the common name or the - oic ending of the IUPAC for the corresponding carboxylic acid is replaced by - amide.
How many carbon atoms are in amide?
Name each compound with the common name, the IUPAC name, or both. This amide has two carbon atoms and is thus derived from acetic acid. The OH of acetic acid is replaced by an NH 2 group. The - ic from acetic (or - oic from ethanoic) is dropped, and - amide is added to give acetamide (or ethanamide in the IUPAC system).
What is an amide functional group?
The amide functional group has an nitrogen atom attached to a carbonyl carbon atom. If the two remaining bonds on the nitrogen atom are attached to hydrogen atoms, the compound is a simple amide. If one or both of the two remaining bonds on the atom are attached to alkyl or aryl groups, the compound is a substituted amide.
Overview
Nomenclature
In the usual nomenclature, one adds the term "amide" to the stem of the parent acid's name. For instance, the amide derived from acetic acid is named acetamide (CH3CONH2). IUPAC recommends ethanamide, but this and related formal names are rarely encountered. When the amide is derived from a primary or secondary amine, the substituents on nitrogen are indicated first in the name. Thus, the amide formed from dimethylamine and acetic acid is N,N-dimethylacet…
Properties
The lone pair of electrons on the nitrogen atom is delocalized into the carbonyl group, thus forming a partial double bond between nitrogen and carbon. In fact the O, C and N atoms have molecular orbitals occupied by delocalized electrons, forming a conjugated system. Consequently, the three bonds of the nitrogen in amides is not pyramidal (as in the amines) but planar. This planar restriction p…
Reactions
Amides undergo many chemical reactions, although they are less reactive than esters. Amides hydrolyse in hot alkali as well as in strong acidic conditions. Acidic conditions yield the carboxylic acid and the ammonium ion while basic hydrolysis yield the carboxylate ion and ammonia. The protonation of the initially generated amine under acidic conditions and the deprotonation of the i…
Synthesis
Many methods exist in amide synthesis.
Amides can be prepared by coupling carboxylic acid with an amine. The direct reaction generally requires high temperatures to drive off the water:
RCO2H + R′R″NH → R′R″NH 2RCO 2 R′R″NH 2RCO 2 → RC(O)NR′R″ + H2O
Many methods involve "activating" the carboxylic acid by converting it to a better
See also
• Amidogen
• Amino radical
• Amidicity
• Metal amides
External links
• IUPAC Compendium of Chemical Terminology