Key Concepts
- An emission (atomic) spectrum is produced when a gas is heated.
- The atoms in the gas absorb energy, causing some electrons to move from the lower energy ground state to a higher energy excited state. ...
- These excited electrons will fall back to the lower energy ground state and emit the energy they had previously absorbed. ...
How atomic spectra are used in the real world?
Uses of Atomic Spectroscopy
- It is used to identify the spectral lines of materials used in metallurgy.
- It is used in pharmaceutical industries to find the traces of materials used.
- It can be used to study multidimensional elements.
- It is used as a tool for studying the structures of atoms and molecules.
What is meant by atomic spectra?
What is Atomic Spectra?
- Characteristics of Atomic Spectrum. The atomic spectrum should be a pure line spectrum. ...
- Atomic Spectrum Overview. In any given set of conditions like pressure, temperature, etc., the collection of all these specific wavelengths is what constitutes the atomic spectrum.
- Rydberg Formula. ...
- Atomic Spectroscopy. ...
- Uses of Atomic Spectroscopy. ...
- Solved Examples. ...
What is the source of atomic emission spectra?
Thus, emission spectra are produced by thin gases in which the atoms do not experience many collisions (because of the low density). The emission lines correspond to photons of discrete energies that are emitted when excited atomic states in the gas make transitions back to lower-lying levels.
How is an atomic emission spectrum of an element produced?
How is the emission spectrum produced? Atomic emission spectra are produced when excited electrons return to the ground state. The emitted light of electrons corresponds to energies of the specific electrons.
What are the three types of atomic spectroscopy?
Atomic spectroscopy is the study of the electromagnetic radiation absorbed or emitted by the atoms. There are three types of atomic spectroscopy and they are: 1 Atomic emission spectroscopy: This involves the transfer of energy from the ground state to an excited state. The electronic transition can be explained in atomic emission. 2 Atomic absorption spectroscopy: For absorption to take place there should be identical energy difference between the lower and higher energy levels. The atomic absorption spectroscopy principle uses the fact that the free electrons generated in an atomizer can absorb radiation at specific frequency. It quantifies the absorption of ground-state atoms in the gaseous state. 3 Atomic fluorescence spectroscopy: This is a combination of atomic emission and atomic absorption as it involves radiation of both excitation and de-excitation.
Why are hydrogen emission lines observed?
The observed spectral lines in the hydrogen emission spectrum are due to the atomic transitions between different energy levels. The spectral series are important in astronomical ...
What is the atomic spectrum?
In any given set of conditions, the collection of all these specific wavelengths is what constitutes the atomic spectrum. Hence, atomic spectra are the spectra of atoms.
What is the study of the electromagnetic radiation absorbed or emitted by the atoms?
Atomic spectroscopy is the study of the electromagnetic radiation absorbed or emitted by the atoms. There are three types of atomic spectroscopy and they are: Atomic emission spectroscopy: This involves the transfer of energy from the ground state to an excited state. The electronic transition can be explained in atomic emission.
What is atomic spectroscopy used for?
Uses of Atomic Spectroscopy. It is used for identifying the spectral lines of materials used in metallurgy. It is used in pharmaceutical industries to find the traces of materials used. It can be used to study multidimensional elements. Stay tuned with BYJU’S to know more about other Physics related concepts.
What is the spectrum of an atom?
The spectrum of the electromagnetic radiation emitted or absorbed by an electron during transitions between different energy level within an atom. When an electron gets excited from one energy level to another, it either emits or absorbs light of a specific wavelength. The collection of all these specific wavelengths ...
What is the atomic structure of an atom?
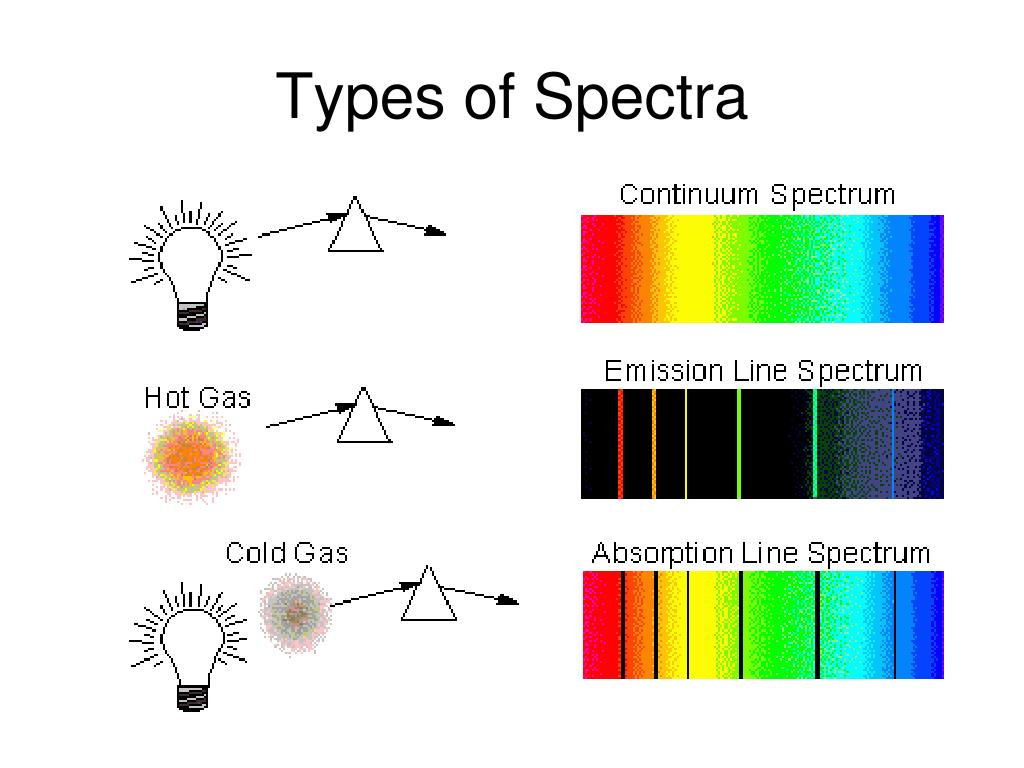
There is an intimate connection between the atomic structure of an atom and its spectral characteristics. Most light is polychromatic and contains light of many wavelengths. Light that has only a single wavelength is monochromatic and is produced by devices called lasers, which use transitions between two atomic energy levels to produce light in a very narrow range of wavelengths. Atoms can also absorb light of certain energies, resulting in a transition from the ground state or a lower-energy excited state to a higher-energy excited state. This produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of an element.
What is the absorption spectrum of an element?
This produces an absorption spectrum, which has dark lines in the same position as the bright lines in the emission spectrum of an element. Atoms of individual elements emit light at only specific wavelengths, producing a line spectrum rather than the continuous spectrum of all wavelengths produced by a hot object.
What is the photon?
The concept of the photon, however, emerged from experimentation with thermal radiation, electromagnetic radiation emitted as the result of a source’s temperature, which produces a continuous spectrum of energies. More direct evidence was needed to verify the quantized nature of electromagnetic radiation.
How old was Balmer when he wrote the paper on hydrogen?
A mathematics teacher at a secondary school for girls in Switzerland, Balmer was 60 years old when he wrote the paper on the spectral lines of hydrogen that made him famous. Balmer published only one other paper on the topic, which appeared when he was 72 years old.
Why do electrons have lines in the spectrum?
Lines in the spectrum were due to transitions in which an electron moved from a higher-energy orbit with a larger radius to a lower-energy orbit with smaller radius. The orbit closest to the nucleus represented the ground state of the atom and was most stable; orbits farther away were higher-energy excited states.
What are the learning objectives of the photoelectric effect?
Learning Objectives. To know the relationship between atomic spectra and the electronic structure of atoms. The photoelectric effect provided indisputable evidence for the existence of the photon and thus the particle-like behavior of electromagnetic radiation.
Who discovered the frequency of hydrogen?
In 1885, a Swiss mathematics teacher, Johann Balmer (1825–1898), showed that the frequencies of the lines observed in the visible region of the spectrum of hydrogen fit a simple equation that can be expressed as follows: where n = 3, 4, 5, 6. As a result, these lines are known as the Balmer series.
The Third Law of Thermodynamics
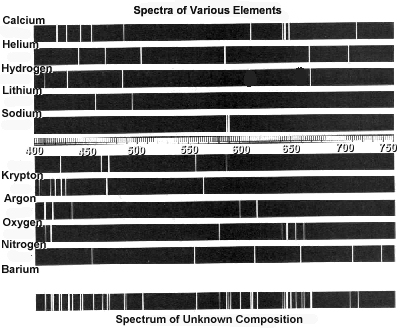
The spectral “lines” of some elements are actually split into groups of several individual lines that are visible at very high resolution. For example, the 412.2 nm “line” of atomic Bi is actually a group of four lines falling within a width of only 0.044 nm.
Spectroscopy and Atomic Physics
The full intricacy of atomic structure and associated transitions is highly specialized. It is not surprising that atomic physics is complicated. One must solve “many-” and “few-” body problems that have no closed-form solutions, even in the classical limit.
Molecular absorption spectrometry in flames and furnaces: A review
Extensive treatments of the theory of molecular spectra are provided in the volume by Welz et al. [5] and in reviews [1–3]. Consequently, only a qualitative overview is provided here. The reader is referred to these references for more detailed and mathematical treatments.
Five years of innovations in development of glow discharges generated in contact with liquids for spectrochemical elemental analysis by optical emission spectrometry
Pawel Pohl, ... Anna Szymczycha-Madeja, in Analytica Chimica Acta, 2021
Atomic Spectroscopy
Atomic spectroscopy is a study of electromagnetic radiation that is absorbed or released from atoms. There are three kinds of atomic spectroscopy, they are;
Uses of Atomic Spectroscopy
It is used in the pharmaceutical industry to find traces of the materials used.
What causes the Balmer series of visible lines for atomic hydrogen?
The Balmer series of visible lines for atomic hydrogen are caused by transitions from the n = 2 orbit to and from higher orbits.
What are the two types of spectra?
Line spectra appear in two forms, absorption spectra, showing dark lines on a bright background, and emission spectra with bright lines on a dark or black background. These two types are in fact related and arise due to quantum mechanical interactions between electrons orbiting atoms and photons of light. Photons of light each have a specific frequency. The energy of a photon is a function of its frequency and is determined by:
What is the transition of electrons in the Bohr model?
As electrons jump down to the n = 2 orbit, they emit photons of specific frequency (hence colour) that can be seen as emission lines in the visible part of the em spectrum. This visible set of lines is called the Balmer series.
What is the blackbody curve?
The continuous spectrum produced by a black body is distinctive and can be shown as an intensity plot of intensity against emitted wavelength. This plot is called the blackbody curve or the Planck curve, after the German physicist Max Planck who first postulated that electromagnetic radiation was quantised.
What happens when an electron orbits a nucleus?
If a photon of a specific frequency interacts with the electron, it can gain sufficient energy to "jump up" one or more levels. The photon is absorbed by the electron so cannot continue on to be detected by an observer.
How can a continuous spectrum be produced?
One means by which a continuous spectrum can be produced is by thermal emission from a black body. This is particularly relevant in astronomy and is discussed in the next section. Astronomical spectra can be combination of absorption and emission lines on a continuous background spectrum. Specific examples are discussed on another page.
What is a black body radiator?
A black body radiator is a theoretical object that is totally absorbent to all thermal energy that falls on it, thus it does not reflect any light so appears black. As it absorbs energy it heats up and re-radiates the energy as electromagnetic radiation.
Definition of Atomic Spectra
The atomic spectra are the spectrum of frequencies of electromagnetic radiation emitted or absorbed during transitions of electrons between energy levels within an atom.
Discovery of Atomic Spectra
The systematic attribution of spectra to chemical elements began in the 1860s with the work of German physicists Robert Bunsen and Gustav Kirchhoff, who found that Fraunhofer lines correspond to spectral emission lines observed in laboratory light sources.
Types of Atomic Spectra
Unlike the spectrum obtained by analyzing the sunlight, the spectra of atoms are not continuous. The spectra of atoms consist of sharp, well-defined lines or bands corresponding to definite frequencies. There are two types of atomic spectra :
Emission Spectrum of Hydrogen Atom
The spectrum of the hydrogen atom can be obtained by passing an electric discharge through the gas taken in the discharge tube under low pressure. The emitted light is analyzed with the help of a spectroscope. The spectrum consists of a large number of lines appearing in different regions of wavelengths.
How do X-rays work?
Like all electromagnetic radiation, X-rays are made of photons. X-ray photons are produced when electrons in the outermost shells of an atom drop to the inner shells. (Hydrogen atoms do not emit X-rays, because the electron energy levels are too closely spaced together to permit the emission of high-frequency radiation.) Transitions of this kind are normally forbidden because the lower states are already filled. However, if an inner shell has a vacancy (an inner electron is missing, perhaps from being knocked away by a high-speed electron), an electron from one of the outer shells can drop in energy to fill the vacancy. The energy gap for such a transition is relatively large, so wavelength of the radiated X-ray photon is relatively short.
How does fluorescence occur?
Fluorescence occurs when an electron in an atom is excited several steps above the ground state by the absorption of a high-energy ultraviolet (UV) photon. Once excited, the electron “de-excites” in two ways. The electron can drop back to the ground state, emitting a photon of the same energy that excited it, or it can drop in a series of smaller steps, emitting several low-energy photons. Some of these photons may be in the visible range. Fluorescent dye in clothes can make colors seem brighter in sunlight by converting UV radiation into visible light. Fluorescent lights are more efficient in converting electrical energy into visible light than incandescent filaments (about four times as efficient). (Figure) shows a scorpion illuminated by a UV lamp. Proteins near the surface of the skin emit a characteristic blue light.
What is the theoretical basis of atomic spectroscopy?
The theoretical basis of atomic spectroscopy is the transition of electrons between energy levels in atoms. For example, if an electron in a hydrogen atom makes a transition from the to the shell, the atom emits a photon with a wavelength. where is energy carried away by the photon and .
How do quantum numbers help us?
Use quantum numbers to estimate the energy, frequency, and wavelength of photons produced by atomic transitions in multi-electron atoms. The study of atomic spectra provides most of our knowledge about atoms. In modern science, atomic spectra are used to identify species of atoms in a range of objects, from distant galaxies to blood samples ...
How to describe radiation?
By the end of this section, you will be able to: 1 Describe the absorption and emission of radiation in terms of atomic energy levels and energy differences 2 Use quantum numbers to estimate the energy, frequency, and wavelength of photons produced by atomic transitions in multi-electron atoms 3 Explain radiation concepts in the context of atomic fluorescence and X-rays
What is the transition between electrons and hydrogen?
An electron transition from the to the shell of a hydro gen atom. To understand atomic transitions in multi-electron atoms, it is necessary to consider many effects, including the Coulomb repulsion between electrons and internal magnetic interactions (spin-orbit and spin-spin couplings).
What type of radiation is produced when an electron collides with another electron?
Braking radiation is not the only type of radiation produced in this interaction. In some cases, an electron collides with another inner-shell electron of a target atom, and knocks the electron out of the atom—billiard ball style.
