Atoms are the basic building blocks of ordinary matter. Atoms can join together to form molecules, which in turn form most of the objects around you. A particular atom will have the same number of protons and electrons and most atoms have at least as many neutrons as protons. What are the tiny building blocks of all matter?
Why are atoms considered the basic building blocks of matter?
Atoms are the basic building blocks of ordinary matter. Atoms can join together to form molecules, which in turn form most of the objects around you. Atoms are composed of particles called protons, electrons and neutrons. Click to see full answer.
Why are atoms called the building blocks of all things?
Well, the basic building blocks that make up matter are called atoms. Sometimes two or more atoms bond, or stick together, and form a molecule. A molecule is the smallest part of a substance that still has all the properties of that substance. For example, a water molecule is made up of two hydrogen atoms and one oxygen atom.
Are atoms really the most basic form of matter?
An atom is the basic unit of matter. The atom is the basic building block of an element, and cannot be broken down further using any chemical means. An atom is made up of three particles: protons, neutrons and electrons. What is the basic unit of matter Brainly? In turn, the atom is the fundamental unit of matter…, that is, of an element.
What are the true building blocks of matter?
Atoms are the basic building blocks of matter. Different elements are made up of different atoms. 2. The arrangement of atoms in a solid, liquid and gas differ from each other. The atoms in a solid are close to each other and orderly arranged.

How do atoms form the building blocks of the entire universe?
Atoms were created after the Big Bang 13.7 billion years ago. As the hot, dense new universe cooled, conditions became suitable for quarks and electrons to form. Quarks came together to form protons and neutrons, and these particles combined into nuclei.
What are the 3 building blocks for atoms?
Atoms are made up of even smaller subatomic particles, three types of which are important: the proton, neutron, and electron. The number of positively-charged protons and non-charged (“neutral”) neutrons, gives mass to the atom, and the number of each in the nucleus of the atom determine the element.
Who said atoms are the building blocks of matter?
One of these philosophers was Democritus (~460 - ~370 B.C.E.), often referred to as the "laughing philosopher" because of his emphasis on cheerfulness (Figure 1.4. 1). He suggested that atomos, or atomon - tiny, indivisible, solid objects - make up all matter in the universe.
Are atoms the building blocks of all matter?
The tiny particles called atoms are the basic building blocks of all matter. Atoms can be combined with other atoms to form molecules. However, they cannot be divided into smaller parts by ordinary means. Each individual atom is made up of smaller particles called electrons, protons and neutrons.
How is an atom made?
An atom itself is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons. The protons and the neutrons make up the center of the atom called the nucleus and the electrons fly around above the nucleus in a small cloud.
What are atoms made of?
Atoms are constructed of two types of elementary particles: electrons and quarks. Electrons occupy a space that surrounds an atom's nucleus. Each electron has an electrical charge of -1. Quarks make up protons and neutrons, which, in turn, make up an atom's nucleus.
Are humans made of atoms?
About 99 percent of your body is made up of atoms of hydrogen, carbon, nitrogen and oxygen. You also contain much smaller amounts of the other elements that are essential for life.
Why are elements called building blocks of matter?
Why are elements called the building blocks of matter? Because all matter is composed of one element or a combination of two or more elements.
What are the building block elements?
There are six main elements that are the fundamental building blocks of life. They are, in order of least to most common: sulfur, phosphorous, oxygen, nitrogen, carbon, and hydrogen.
What does building blocks mean in chemistry?
Building block is a term in chemistry which is used to describe a virtual molecular fragment or a real chemical compound the molecules of which possess reactive functional groups.
What are atoms made of?
Atoms are constructed of two types of elementary particles: electrons and quarks. Electrons occupy a space that surrounds an atom's nucleus. Each electron has an electrical charge of -1. Quarks make up protons and neutrons, which, in turn, make up an atom's nucleus.
What are the building blocks of proteins?
The building blocks of proteins are amino acids, which are small organic molecules that consist of an alpha (central) carbon atom linked to an amino group, a carboxyl group, a hydrogen atom, and a variable component called a side chain (see below).
What is the substance of the universe?
The substance of the universe—from a grain of sand to a star —is called matter. Scientists define matter as anything that occupies space and has mass. An object’s mass and its weight are related concepts, but not quite the same. An object’s mass is the amount of matter contained in the object, and the object’s mass is the same whether ...
How are elements arranged in the periodic table?
The elements are arranged in order of their atomic number, with hydrogen and helium at the top of the table, and the more massive elements below. The periodic table is a useful device because for each element, it identifies the chemical symbol, the atomic number, and the mass number, while organizing elements according to their propensity to react with other elements. The number of protons and electrons in an element are equal. The number of protons and neutrons may be equal for some elements, but are not equal for all.
Why is helium unlikely to react with other atoms?
Helium, as well as larger atoms with eight electrons in their valence shell, is unlikely to participate in chemical reactions because they are stable. All other atoms tend to accept, donate, or share electrons in a process that brings the electrons in their valence shell to eight (or in the case of hydrogen, to two).
How many protons does carbon have?
The answer is the unique quantity of protons each contains. Carbon by definition is an element whose atoms contain six protons. No other element has exactly six protons in its atoms. Moreover, all atoms of carbon, whether found in your liver or in a lump of coal, contain six protons.
Which element has only one electron?
Electrons orbit the atomic nucleus at distinct levels of energy called electron shells. (a) With one electron, hydrogen only half-fills its electron shell. Helium also has a single shell, but its two electrons completely fill it. (b) The electrons of carbon completely fill its first electron shell, but only half-fills its second. (c) Neon, an element that does not occur in the body, has 10 electrons, filling both of its electron shells.
How do radioisotopes work?
Radioisotopes emit subatomic particles that can be detected and tracked by imaging technologies. One of the most advanced uses of radioisotopes in medicine is the positron emission tomography (PET) scanner, which detects the activity in the body of a very small injection of radioactive glucose, the simple sugar that cells use for energy. The PET camera reveals to the medical team which of the patient’s tissues are taking up the most glucose. Thus, the most metabolically active tissues show up as bright “hot spots” on the images ( [link] ). PET can reveal some cancerous masses because cancer cells consume glucose at a high rate to fuel their rapid reproduction.
Why is the periodic table useful?
The periodic table is a useful device because for each element, it identifies the chemical symbol, the atomic number, and the mass number, while organizing elements according to their propensity to react with other elements. The number of protons and electrons in an element are equal.
What are the three subatomic particles that make up an atom?
Atoms are made up of even smaller subatomic particles, three types of which are important: the proton , neutron, and electron. The number of positively-charged protons and non-charged (“neutral”) neutrons, gives mass to the atom, and the number of each in the nucleus of the atom determine the element. The number of negatively-charged electrons that “spin” around the nucleus at close to the speed of light equals the number of protons. An electron has about 1/2000th the mass of a proton or neutron.
How many protons does carbon have?
Although each element has a unique number of protons, it can exist as different isotopes. An isotope is one of the different forms of an element, distinguished from one another by different numbers of neutrons. The standard isotope of carbon is 12 C, commonly called carbon twelve. 12 C has six protons and six neutrons, for a mass number of twelve. All of the isotopes of carbon have the same number of protons; therefore, 13 C has seven neutrons, and 14 C has eight neutrons. The different isotopes of an element can also be indicated with the mass number hyphenated (for example, C-12 instead of 12 C). Hydrogen has three common isotopes, shown in Figure 3.
What is the smallest quantity of an element that retains the unique properties of that element?
An atom is the smallest quantity of an element that retains the unique properties of that element. In other words, an atom of hydrogen is a unit of hydrogen—the smallest amount of hydrogen that can exist. As you might guess, atoms are almost unfathomably small. The period at the end of this sentence is millions of atoms wide.
Do atoms exist as independent entities?
In the human body, atoms do not exist as independent entities. Rather, they are constantly reacting with other atoms to form and to break down more complex substances. To fully understand anatomy and physiology you must grasp how atoms participate in such reactions. The key is understanding the behavior of electrons.
What is a molecule in chemistry?
Moleculeis a very important word in chemistry. A molecule is two or more atoms that have chemically bonded with each other. The atoms in a molecule can be of the same kind (in which case it would be a molecule of an element), or they can be of different kinds (in which case it would be a molecule of a compound).
What are diatomic molecules?
Oxygen (O2), nitrogen (N2), hydrogen (H2), chlorine (Cl2) and some other elements from the non-metals all form diatomic molecules. Draw a picture of one of these diatomic molecules in the space below. Learners must draw two circles joint to each other that are of the same size and colour.
What is the term for a chemical reaction in which a compound is broken down into simpler compounds and even elements?
A chemical reaction in which a compound is broken down into simpler compounds and even elements, is called a decomposition reaction. Compounds cannot be separated by physical processes, but they can be separated into their elements (or simpler compounds) by chemical processes. Mixtures.
What are some examples of compounds?
Water (H2O), carbon dioxide (CO2) and salt or sodium chloride (NaCl) are examples of compounds, while oxygen gas (O2), hydrogen gas (H2) and nitrogen gas (N2) are examples of elements. The compound with the formula H2O2also consists of hydrogen atoms and oxygen atoms.
What letter does not appear on the periodic table?
The only letter that does not appear on the Periodic Table is the letter 'J'. The elements are arranged in order of increasing atomic number. You can find a larger version of the Periodic Table on the inside cover of your book for easy reference. A quick revision of the Periodic Table of Elements.
Where are electrons found?
They are also found in the atomic nucleus. Electrons are negatively charged particles, much smaller than protons and neutrons. A cloud of fast-moving electrons surrounds the atomic nucleus. In a neutral atom, the number of protons always equals the number of electrons; hence the atom is neutral.
Can you see an atom with the naked eye?
A model of the atom. Atoms cannot be seen with the naked eye, only with very powerful microscopes. However, scientists have a good idea of how they behave in different situations. Based on these ideas, they have developed a model of what the atom looks like, to help us understand atoms better.
How many blocks are there in the atom diagram?
Each of the 15 blocks contains a diagram representing atoms and molecules of matter.
Which subatomic particles determine the structure of an atom?
The three main sub-atomic particles that determine the structure of the atom are protons, neutrons and electrons.
How much would a nucleus weigh?
If the nucleus was scaled up to the size of a full stop, it would weigh as much as a fully loaded minibus taxi, or 2,5 tonnes!
Where are electrons found?
They are also found in the atomic nucleus. Electrons are negatively charged particles, much smaller than protons and neutrons. A cloud of fast-moving electrons surrounds the atomic nucleus. In a neutral atom, the number of protons always equals the number of electrons; hence the atom is neutral.
Which subatomic particles are smaller than protons?
Electrons are the smallest of the three sub-atomic particles. Electrons are about 2000 times smaller than protons and neutrons. The electrons move in a zone around the atomic nucleus at extremely high speeds, forming an electron cloud that is much larger than the nucleus. Have a look again at the drawing which shows a model of the atom to see this.
Why do magnets stick together?
Learners may say that magnets stick together because they attract each other. Point out to them that magnets will indeed attract each other if they are lined up correctly. Magnets can also repel each other if they are lined up differently. Learners will look more at magnetic forces in Gr. 9 Energy and Change.
What letter does not appear on the periodic table?
The only letter that does not appear on the Periodic Table is the letter 'J'. The elements are arranged in order of increasing atomic number. You can find a larger version of the Periodic Table on the inside cover of your book for easy reference. A quick revision of the Periodic Table of Elements.
What are the building blocks of matter?
Well, the basic building blocks that make up matter are called atoms. Sometimes two or more atoms bond, or stick together, and form a molecule. A molecule is the smallest part of a substance that still has all the properties of that substance. For example, a water molecule is made up of two hydrogen atoms and one oxygen atom.
What do students learn about atoms?
Students use the associated activity to learn about atoms and their structure (protons, electrons, neutrons) — the building blocks of matter. They see how scientific discoveries about atoms and molecules influence new technologies developed by engineers.
Where are the protons and neutrons in an atom?
The three main ones are protons and neutrons, which are found in the nucleus or core of the atom, and electrons, which exist outside of the nucleus. Refer to the Gumdrop Atoms activity to illustrate the anatomy of an atom to give students a better understanding of how these subatomoic particles interact.
Which electrons are most easily shared with or transferred to other atoms?
Generally, the number of protons and electrons balance out to make the atom have an electrically neutral charge. Electrons that are farthest away from the nucleus of an atom (valence electrons) are the ones that are most easily shared with or transferred to other atoms.
How many atoms are in a substance?
Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. (Grades 6 - 8) More Details
Where are electrons found in an atom?
True or False: Electrons are found in the nucleus of an atom. (Answer: False; electrons are found in shells around the outside of the nucleus .) True or False: Engineers use their knowledge of atoms and molecules to develop new technologies. (Answer: True) True or False: Lasers are only used in science laboratories.
Which particle is most easily changed?
Normally, though, the number of electrons is the particle that is most easily changed, because of its lower bonding energy.
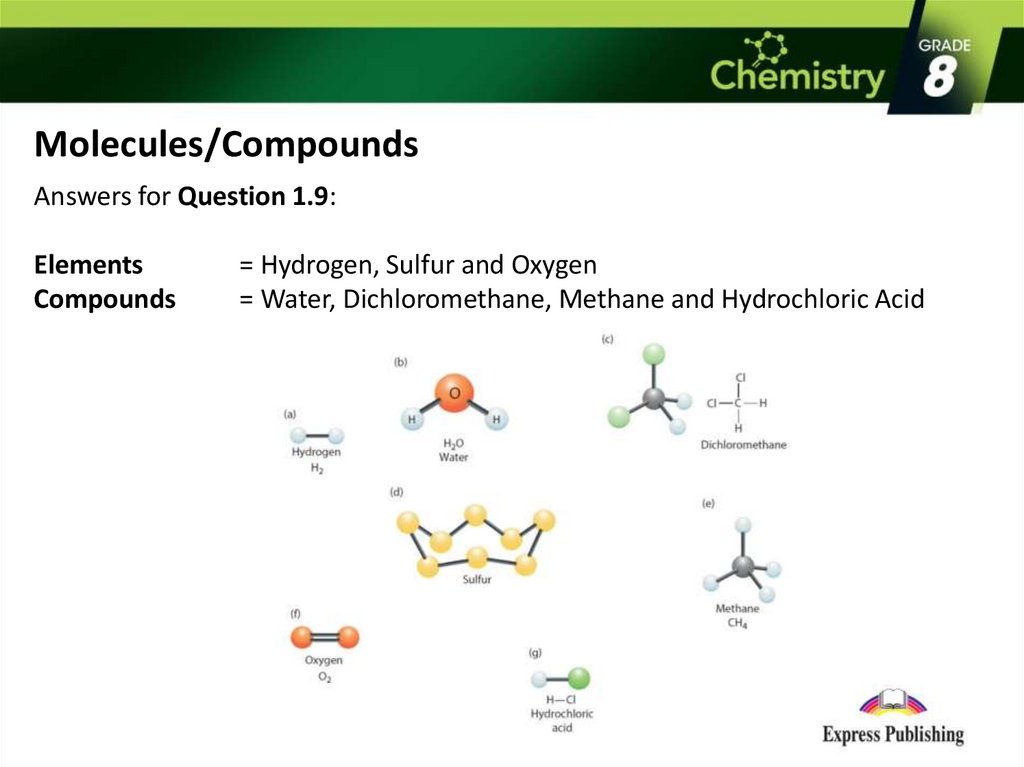
Elements and Compounds
Atoms and Subatomic Particles
- An atomis the smallest quantity of an element that retains the unique properties of that element. In other words, an atom of hydrogen is a unit of hydrogen—the smallest amount of hydrogen that can exist. As you might guess, atoms are almost unfathomably small. The period at the end of this sentence is millions of atoms wide.
Isotopes
- Although each element has a unique number of protons, it can exist as different isotopes. An isotope is one of the different forms of an element, distinguished from one another by different numbers of neutrons. The standard isotope of carbon is 12C, commonly called carbon twelve. 12C has six protons and six neutrons, for a mass number of twelve. All of the isotopes o…
The Behavior of Electrons
- In the human body, atoms do not exist as independent entities. Rather, they are constantly reacting with other atoms to form and to break down more complex substances. To fully understand anatomy and physiology you must grasp how atoms participate in such reactions. The key is understanding the behavior of electrons. Although electrons do not follow rigid orbits a se…