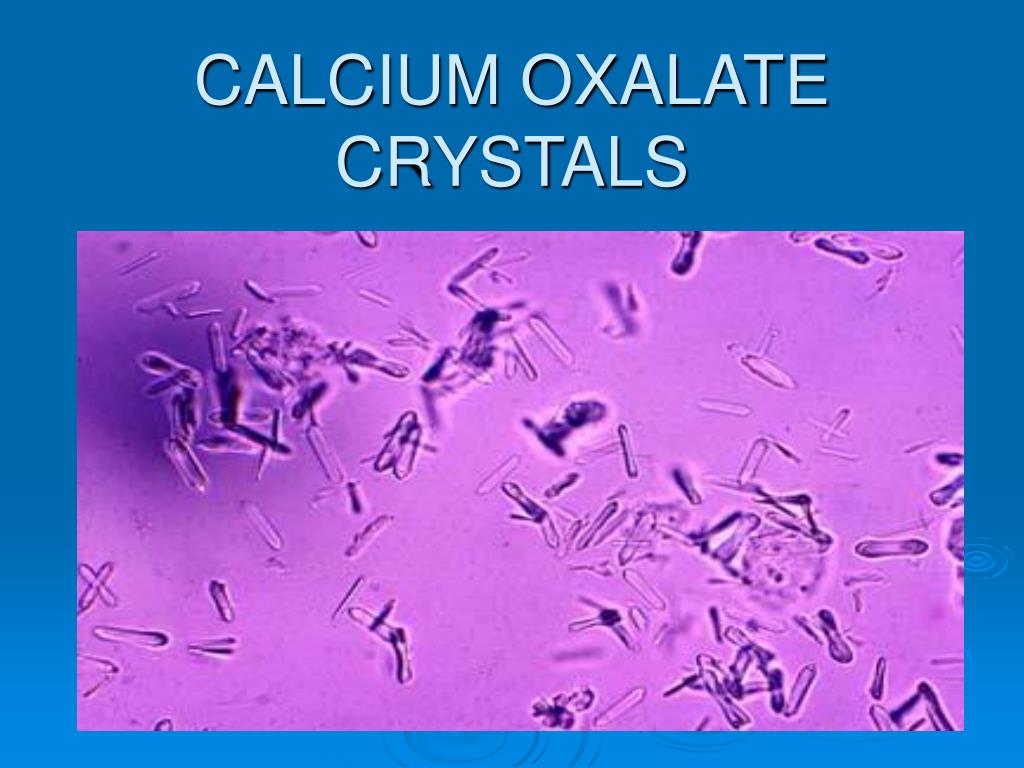
What do you need to know about calcium oxalate crystals?
- Urine tests to measure levels of oxalate and other specific enzymes; urine is also checked for crystals
- Blood test to measure the amount of oxalate in blood.
- Scans (X-rays, ultrasound, and/or CT) of the kidneys and urinary tract to check for kidney stones or calcium oxalate crystals.
What is the formula for calcium oxalate?
Calcium oxalate in archaic terminology, oxalate of lime is a calcium salt of oxalate with the chemical formula CaC2O4 H2O x, where x can vary. All forms are colorless or white.
Is calcium found in plants?
The calcium is usually taken up by the plant in the form of Ca ++ form which you should be already aware of. Calcium concentration in the plants ranges between 0.2 - 1.0 per cent . The calcium taken up by plants are mainly from sources such as lime, dolomite, gypsum, feldspar, amphiboles etc.
What are calcium oxalate stones?
Urinary stones are predominantly composed of calcium oxalate (CaOx), mostly formed in the kidneys, and highly recurrent. Although kidney stone is widely perceived as a not life-threatening condition, it has a lethal consequence. Stones progressively damage ...

How are calcium oxalate crystals formed?
After your body uses what it needs, waste products travel through the bloodstream to the kidneys and are removed through urine. Urine has various wastes in it. If there is too much waste in too little liquid, crystals can begin to form. These crystals may stick together and form a solid mass (a kidney stone).
How are crystal in plants formed?
In angiosperms crystal formation is generally intracellular and crystals form inside the vacuoles of specialized cells called idioblast. However, in gymnosperms most of the crystals form in the cell wall (Kinzel 1989).
Why do plants produce calcium oxalate?
Major functions of CaOx crystal formation in plants include high-capacity calcium (Ca) regulation and protection against herbivory.
Where are plant crystals formed?
These plants accumulate CaOx through a biomineralization process in diverse ways. It has been described that these crystals are formed mainly in the intra-vacuolar membrane or in the chamber of specialized crystalline cells known as idioblasts [44,45,46,47,48] (Figure 1).
What are 4 major processes for how crystals form?
The four main categories of mineral formation are: (1) igneous, or magmatic, in which minerals crystallize from a melt, (2) sedimentary, in which minerals are the result of sedimentation, a process whose raw materials are particles from other rocks that have undergone weathering or erosion, (3) metamorphic, in which ...
Where are calcium oxalate crystals found?
Calcium oxalate is a common biomineral in plants, occurring as crystals of various shapes. It can be found in any tissue or organ in plants and is often formed in the vacuoles of specialized cells called crystal idioblasts.
Where does calcium come from for plants?
Calcium is acquired from the soil solution by the root system and translocated to the shoot via the xylem. The Ca flux to the xylem is high, and a rate of 40 nmol Ca h–1 g–1 f. wt root is not unreasonable in an actively growing plant (White, 1998).
Do plants excrete calcium oxalate?
Plants do not have an excretory system, and so have no way to eliminate excess calcium other than by eliminating the cells, tissues or organs in which it is stored. Therefore, the elimination of excess calcium emerges as a relevant function of CaOx crystals for plants.
Why do plants produce crystal?
Many plant species produce crystal inclusions as a defense mechanism against herbivory. Most crystalline aggregations, or druse crystals, found in plants are made of calcium oxalate, which is the compound that most frequently forms kidney stones in humans.
What are 3 ways crystals are formed?
You can grow crystals in one of three major ways: from a vapor, from a solution or from melt.
What plants contain calcium oxalate crystals?
Oxalate plants contain sharp, tiny crystals in their juices, leaves and stems, called calcium oxalate crystals....Examples of plants that contain oxalates include:Caladium.Calla Lily.Devil's Ivy.Dieffenbachia (Dumb Cane)Elephant's Ear.
Which plant has calcium oxalate crystals?
Calcium Oxalate Crystals in Leaves of the Extremophile Plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae)
Why do plants produce crystal?
Many plant species produce crystal inclusions as a defense mechanism against herbivory. Most crystalline aggregations, or druse crystals, found in plants are made of calcium oxalate, which is the compound that most frequently forms kidney stones in humans.
What are 3 ways crystals are formed?
You can grow crystals in one of three major ways: from a vapor, from a solution or from melt.
What are the oxalates in plants?
Many plants contain oxalates. Some oxalates, such as sodium and potassium oxalates, are water soluble, and others form insoluble crystals, such as calcium and magnesium oxalates. Calcium oxalates crystals have been found in more than 200 plant families and in almost all types of tissues of these plants, including leaf, stem, root and even anther.
What vegetables are raphide containing?
Other cases involved different types of vegetables, such as water spinach, Chinese white cabbage, Chinese flowering cabbage and watercress. These vegetables are not supposed to contain calcium oxalate raphides, and it is postulated that the vegetables might have been mixed with small amount of raphide-containing plants and consumed. In some of these incidents, leaves of wild taro may have been used to wrap or cover vegetables during transportation and processing.
Why does taro taste so bad after cooking?
Some raphides even have grooves which hold these enzymes. This is the reason why the acrid taste of raw edible taro is substantially reduced after cooking, as cooking destroys the enzymes. However, cooking cannot remove the poisoning effect of wild taro and it should not be consumed.
How to prevent calcium oxalate poisoning?
The most effective way to prevent calcium oxalate poisoning is not to consume plants that are not known to be edible, and to avoid their contamination to edible vegetables. Figure 2: Leaf of wild taro, which is known to contain calcium oxalate raphides.
What is the most irritating shape of calcium oxalate?
It is believed that the needle-shaped calci um oxalate is the most irritating, while other shapes are less likely to inflict damage. The calcium oxalate crystals of wild taro are needle-shaped, compared with those block-shaped ones found in spinach. Therefore, although calcium oxalate crystals are present in both wild taro and spinach, ...
What causes sharp needles in plants?
The sharp needle-shaped raphides are packed in bundles within plant cells. Damage to the plant cells during chewing causes water to enter and swell up the cells. This stimulates the forceful propulsion of the sharp raphides into the surrounding environment, stabbing the sensitive tissues of the tongue, gums and throat and resulting in tissue injuries in the oral cavity.
What to remove from a vegetable garden?
Remove any unidentifi ed plants and objects mixed with vegetables.
What is Oxystelma esculentum?
Oxystelma esculentum (L. f.) Sm. (Apocynaceae) is a perennial medicinal climber, enormously explored in traditional and modern systems of medicines. Due to over-harvesting from the wild, this species is categorized as ‘rare’. Hence, this study was aimed to develop an in vitro regeneration system for O. esculentum as a conservation measure. Nodal explants were cultured on Murashige and Skoog (MS) medium supplemented with cytokinins [benzyladenine (BA) and meta-Topolin (mT)] for the establishment of cultures. The growth of shoots from the pre-existing meristems of explants was obtained on MS medium containing 4.14 µM mT with the highest mean number (7.25) of shoots. The maximum shoot proliferation frequency (124.8 shoots) was achieved on MS medium containing 2.07 µM mT and 1.42 µM indole-3-acetic acid (IAA). The foliar micro-morpho-anatomical stability of in vitro raised shoots (from BA and mT) was analyzed using light microscopy. Although the in vitro raised leaves possessed microscopic anomalies, the BA-derived leaves showed a higher degree of abnormalities, such as underdeveloped stomata with single guard cells, reduced vein and trichome density, poor deposition of cutin, and less differentiation of ground and vascular tissue systems. Comparatively, mT-derived in vitro leaves had functional stomata, a higher amount of cutin deposition, and remarkable developments in vascular and ground tissue systems. The regenerated shoots were rooted following pulse treating with 976.08 µM indole-3-butyric acid (IBA) under greenhouse (ex vitro) conditions. All the mT raised plantlets (100%) were survived in the field conditions. This study could suggest a highly efficient cytokinin (mT) for large-scale propagation and conservation of O. esculentum, which could ultimately help in the production of the quality shoots in terms of essential structural developments for successful rooting and hardening processes.
What is the process of kidney stones?
GeoBioMed — a new transdisciplinary approach that integrates the fields of geology, biology and medicine — reveals that kidney stones composed of calcium-rich minerals precipitate from a continuum of repeated events of crystallization, dissolution and recrystallization that result from the same fundamental natural processes that have governed billions of years of biomineralization on Earth. This contextual change in our understanding of renal stone formation opens fundamentally new avenues of human kidney stone investigation that include analyses of crystalline structure and stratigraphy, diagenetic phase transitions, and paragenetic sequences across broad length scales from hundreds of nanometres to centimetres (five Powers of 10). This paradigm shift has also enabled the development of a new kidney stone classification scheme according to thermodynamic energetics and crystalline architecture. Evidence suggests that ≥50% of the total volume of individual stones have undergone repeated in vivo dissolution and recrystallization. Amorphous calcium phosphate and hydroxyapatite spherules coalesce to form planar concentric zoning and sector zones that indicate disequilibrium precipitation. In addition, calcium oxalate dihydrate and calcium oxalate monohydrate crystal aggregates exhibit high-frequency organic-matter-rich and mineral-rich nanolayering that is orders of magnitude higher than layering observed in analogous coral reef, Roman aqueduct, cave, deep subsurface and hot-spring deposits. This higher frequency nanolayering represents the unique microenvironment of the kidney in which potent crystallization promoters and inhibitors are working in opposition. These GeoBioMed insights identify previously unexplored strategies for development and testing of new clinical therapies for the prevention and treatment of kidney stones.
Where are sloths found in Texas?
We present new information about the Late Pleistocene Shasta ground sloth (Nothrotheriops shastensis). Spirit Eye Cave in the Sierra Vieja along the Rio Grande provides the newest evidence that the Shasta ground sloth inhabited further south in the mountains of the southwestern Trans-Pecos, Texas, than has been previously documented. The cave is one of only twelve known Nothrotheriops dung localities. During excavation of the cave, packrat middens and sloth dung were discovered. Two areas within the cave provide radiocarbon dated ground sloth dung and packrat midden macrobotanical remains which permit the reconstruction of the sloth diet and local biotic habitat at 30,800 and 12,900 calibrated YBP. The local community at 30,800 calibrated years ago was a pinyon-juniper woodland with yucca, sandpaper bush, globemallow, cactus, and barberry in the understory based on the packrat midden from the cave. The dung contents indicate that the diet of the sloth included C3 and C4 grasses along with Agave. Data for the local vegetation community and sloth diet from 12,900 years ago indicate that during this late glacial time, the region was still a pinyon-juniper woodland but also contained Celtis, Quercus, and Larrea, among other taxa.
Where do calcium oxalate crystals form?
Calcium (Ca) oxalate crystals occur in many plant species and in most organs and tissues. They generally form within cells although extracellular crystals have been reported. The crystal cells or idioblasts display ultrastructural modifications which are related to crystal precipitation. Crystal formation is usually associated with membranes, chambers, or inclusions found within the cell vacuole (s). Tubules, modified plastids and enlarged nuclei also have been reported in crystal idioblasts. The Ca oxalate crystals consist of either the monohydrate whewellite form, or the dihydrate weddellite form. A number of techniques exist for the identification of calcium oxalate. X-ray diffraction, Raman microprobe analysis and infrared spectroscopy are the most accurate. Many plant crystals assumed to be Ca oxalate have never been positively identified as such. In some instances, crystals have been classified as whewellite or weddellite solely on the basis of their shape. Certain evidence indicates that crystal shape may be independent of hydration form of Ca oxalate and that the vacuole crystal chamber membranes may act to mold crystal shape; however, the actual mechanism controlling shape is unknown. Oxalic acid is formed via several major pathways. In plants, glycolate can be converted to oxalic acid. The oxidation occurs in two steps with glyoxylic acid as an intermediate and glycolic acid oxidase as the enzyme. Glyoxylic acid may be derived from enzymatic cleavage of isocitric acid. Oxaloacetate also can be split to form oxalate and acetate. Another significant precursor of oxalate in plants is L-ascorbic acid. The intermediate steps in the conversion of L-ascorbic acid to oxalate are not well defined. Oxalic acid formation in animals occurs by similar pathways and Ca oxalate crystals may be produced under certain conditions. Various functions have been attributed to plant crystal idioblasts and crystals. There is evidence that oxalate synthesis is related to ionic balance. Plant crystals thus may be a manifestation of an effort to maintain an ionic equilibrium. In many plants oxalate is metabolized very slowly or not at all and is considered to be an end product of metabolism. Plant crystal idioblasts may function as a means of removing the oxalate which may otherwise accumulate in toxic quantities. Idioblast formation is dependent on the availability of both Ca and oxalate. Under Ca stress conditions, however, crystals may be reabsorbed indicating a storage function for the idioblasts for Ca. In addition, it has been suggested that the crystals serve purely as structural supports or as a protective device against foraging animals. The purpose of this review is to present an overview of plant crystal idioblasts and Ca oxalate crystals and to include the most recent literature.
Is Bruguiera cylindrica tolerant to NaCl?
A hydroponic experiment in NaCl tolerant mangrove, Bruguiera cylindrica plantlets was done to investigate the interactive effects of CdCl2+NaCl as well as the individual effects of CdCl2. CdCl2 alone results in enhanced level of cell membrane lipid peroxidation, damaging normal structure, and function of the membrane and there by leading to reduction of membrane stability index and thus severely promoting the electrolyte leakage than the combined treatment of CdCl2+NaCl. Antioxidants greatly contribute to cope with the stress by scavenging ROS and were more prominent in individual stress than combined stress. B. cylindrica can be categorized as a highly tolerant mangrove species with a Tolerance Index (TI) of above 60% toward CdCl2+NaCl (TI is 70 and 75% in leaf and root). But TI was only 63 and 67% in leaf and root, respectively, on exposure to CdCl2 alone indicating that this plant is less tolerant to Cd alone. The existence of B. cylindrica depends on the heavy metals being introduced into the mangrove ecosystem and imparts a combination stress along with NaCl. In this regard, B. cylindrica survives up to 0.20 mM CdCl2 along with NaCl. Thus, the high NaCl tolerance mechanisms in this mangrove species could be equally efficient in imparting tolerance toward high concentration of Cd along with NaCl and exhibits phytostabilization in polluted coastal wetlands.
How does calcium oxalate affect morphology?
Studies of calcium oxalate crystallization have identified other factors that influence morphology. For example, relative concentrations of calcium and oxalate affected hydration of crystals produced in vitro ( Frey-Wyssling, 1981 ). When oxalate was introduced into a concentrated solution of calcium, crystals of calcium oxalate dihydrate predominated, whereas calcium introduced into a concentrated solution of oxalate resulted in the monohydrate form. The order in which calcium and oxalate ions are imported into crystal chambers during development and relative concentrations of each ion within the chambers could thus have significant effects on resulting crystal morphology. Other studies of calcium oxalate crystallization in vitro ( Cody and Horner, 1984; Cody and Cody, 1987) have demonstrated the potential of a wide variety of additives to alter crystal morphology so as to produce crystals that mimic certain morphologies made by plants.
What are druses in plants?
The druses are spherical aggregates of calcium oxalate that form in mesophyll cells. (C) Isolated bundle of raphides, needle-shaped crystals, from leaves of grape ( Vitis labrusca ). Several hundred raphides form per cell in idioblasts that are distributed throughout the plant.
What are the two types of crystals in plants?
Plant crystals display an astonishing variety of morphologies, most of which conform to one of the following categories defined by botanists ( Franceschi and Horner, 1980 ): (1) prisms, consisting of simple regular prismatic shapes; (2) druses, which are spherical aggregates of crystals; (3) styloids, acicular crystals that form singly; (4) raphides, acicular crystals that form in bundles; and (5) crystal sand, small tetrahedral crystals that form in clusters. Calcium oxalate exists in two chemical forms, monohydrate and dihydrate, and both of these occur in plants ( Arnott et al., 1965; Frey-Wyssling, 1981 ). The observed morphologies represent elaborations and modifications of basic crystal structure for either the monohydrate or dihydrate form. The monohydrate is more stable and is more commonly found in plants than is the dihydrate.
Why is it important to understand the nature and ontogeny of vacuolar constituents associated with crystal formation?
Characterizing the nature and ontogeny of vacuolar constituents associated with crystal formation and understanding how these specialized vacuoles differ from normal vacuoles are essential for clarifying the mechanisms of cellular control over the crystallization process. This review emphasizes, in particular, questions about the structure, composition, and function of crystal chambers in calcium oxalate crystallization.
What is the role of macromolecules in biomineralization?
In most biomineralization processes, specialized cells and/or organic macromolecules in or around specialized cells govern and mediate crystal formation . These cells and their associated molecules, collectively termed the organic matrix, function in a variety of ways to compartmentalize the crystallization process, to nucleate crystals, and to modify crystal growth and morphology ( Lowenstam and Weiner, 1989; Simkiss and Wilbur, 1989 ).
Where does calcium oxalate crystallize?
In higher plants, calcium oxalate typically develops within intravacuolar membrane chambers of specialized cells. The complex cellular features associated with calcium oxalate crystallization indicate that it constitutes a biologically controlled process, analogous to calcification processes that shape bones, teeth, and shells in animals ( Arnott, 1966 ). This review addresses key questions about cell-mediated crystallization of calcium oxalate in plants, including how and why plant cells make crystals, and discusses important cellular, developmental, and physiological aspects of this phenomenon. The broader study of biomineralization provides a context for understanding calcium oxalate crystallization in plants.
Where does calcium oxalate form?
Calcium oxalate crystals may form in any organ or tissue within plants. For example, crystals occur in roots, stems, leaves, flowers, fruits, and seeds ( Franceschi and Horner, 1980) and within epidermal ( Zindler-Frank, 1975 ), ground ( Horner and Whitmoyer, 1972 ), and vascular ( Wang et al., 1994) tissues. Calcium oxalate often forms in idioblasts, cells that develop in isolation with structure or content distinct from surrounding cells ( Foster, 1956 ). In other instances, crystals may develop in defined groups of cells, as in files of bundle sheath cells ( Borchert, 1984 ), for example, or in a single layer of the seed coat ( Webb and Arnott, 1982 ). Less often, entire tissues such as endosperm ( Spitzer and Lott, 1982) or leaf epidermis ( Brubaker and Horner, 1989) accumulate calcium oxalate in every cell or in a majority of cells.
Where are calcium oxalate crystals produced in Peperomia?
The observation that calcium oxalate crystals in Peperomia are specifically produced in the photosynthetic palisade cells rather than in specialized idioblasts indicates a function other than calcium regulation in these species.
Where is calcium oxalate found in plants?
Cell biology. Biochemistry. Calcium oxalate is a common biomineral in plants, occurring as crystals of various shapes. It can be found in any tissue or organ in plants and is often formed in the vacuoles of specialized cells called crystal idioblasts.
What are the cells that plants have?
Many plants have specialized cells, called crystal idioblasts, that contain single or multiple needle-like crystals that appear to serve a primary function in bulk regulation of calcium in tissues and a secondary function in defense against grazing animals.