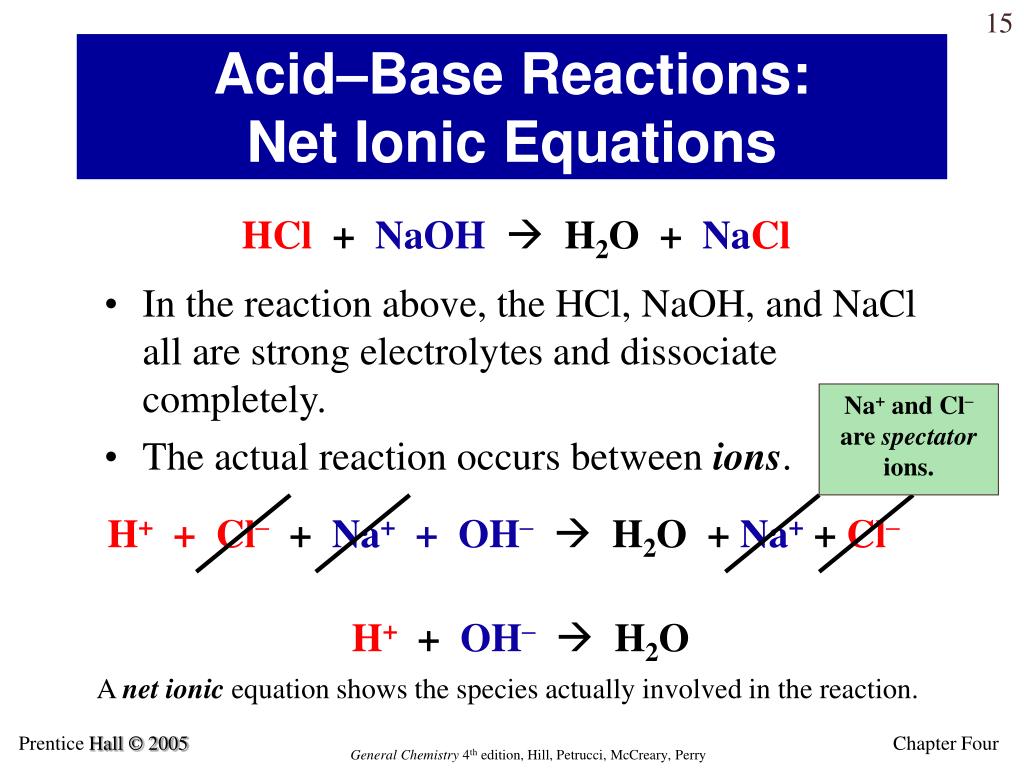
The difference between Complete ionic and net ionic equation is that complete ionic equation gives all the ionic species participated in the chemical reaction whereas net ionic reaction gives the chemical species participated in the formation of the final product. What is the difference between a molecular equation and a chemical equation?
How do you write a complete ionic equation?
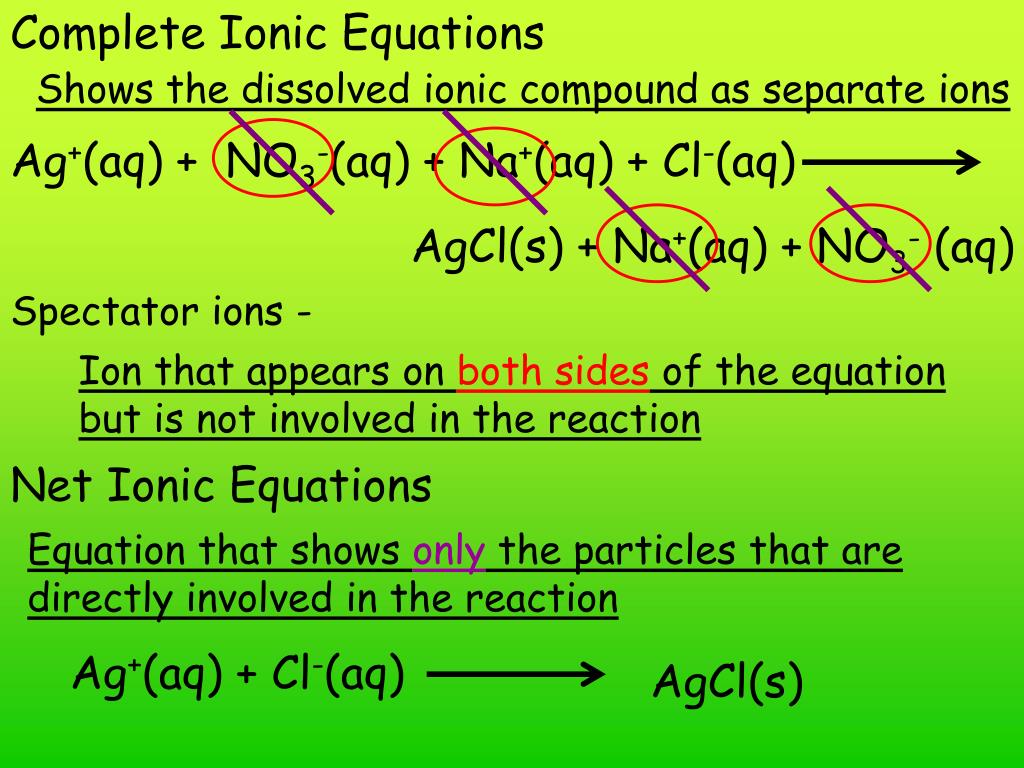
written as a ‘complete’ (meaning with all ions shown) ionic reaction becomes: Ag⁺ (aq) + NO₃⁻ (aq) + Na⁺ (aq) + Cl⁻ (aq) → AgCl (s) + Na⁺ (aq) + NO₃⁻ (aq) So, to write a complete ionic reaction, you have to show all parts of dissociated molecules as ions. The characterizatio Continue Reading Rahim Marwat , lives in Pakistan (1986-present)
What are the rules for writing an ionic equation?
Rules for Writing Net Ionic Equations. All reactions occur- no reversible reactions. All equations must be written as net ionic equations, that is, only the species undergoing change in the reaction are shown. Spectator ions must not appear. All ionic species must be written in their ionic form with the .
How do you calculate an ionic equation?
Part 2 Part 2 of 2: Writing a Net Ionic Equation
- Balance the complete molecular equation. Before writing a net ionic equation, you must first make sure your starting equation is completely balanced.
- Identify the states of matter of each compound in the equation. ...
- Determine what species will dissociate (separate into cations and anions) in solution. ...
- Calculate the charge of each dissociated ion. ...
What does a complete ionic equation give?
The two most common forms of ionic equations are complete ionic equations and net ionic equations. The complete ionic equation indicates all of the dissociated ions in a chemical reaction. The net ionic equation cancels out ions that appear on both sides of the reaction arrow because they essentially don't participate in the reaction of interest.
What is the difference between chemical equations and net ionic equations?
The overall chemical equation shows all the substances present in their undissociated forms; the complete ionic equation shows all the substances present in the form in which they actually exist in solution; and the net ionic equation is derived from the complete ionic equation by omitting all spectator ions, ions that ...
What does a complete ionic equation show?
Complete ionic equations show dissolved ionic solids as separated ions. Net ionic equations show only the ions and other substances that change in a chemical reaction.
What is a net ionic equation in chemistry?
net ionic equation: a chemical equation in which only those ions undergoing chemical changes during the course of the reaction are represented.
How do you complete ionic equations?
Write the ionic equation by breaking all the soluble ionic compounds (those marked with an (aq)) into their respective ions. Each ion should be shown with its charge and an (aq) to show that it is present in solution. Use coefficients to show the number of each ion present.
What information does the complete ionic equation give apex?
Complete ionic equations are ionic equations in which strong acids and soluble ionic compounds are expressed in the form of dissociated ions. Thus, the complete ionic equation provides information regarding all of the substances present in the solution along with the actual form in which they exist in the solution.
Is a complete ionic equation more accurate than a molecular equation?
Complete Ionic Equation Represents all solution phase reactants and products that are strong electrolytes as ions. This is a more accurate representation of what actually exists in the solution than the molecular equation shown above.
What is an ionic equation GCSE?
Ionic equations are chemical equations that show only ions that participate in a chemical reaction. In other words, the ions that react together in solution and form new substances. The other ions that don't participate are called spectator ions.
Why are net ionic equations important?
Net Ionic Equations Are Important One of the most useful applications of the concept of principal species is in writing net ionic equations. These are equations that focus on the principal substances and ions involved in a reaction--the principal species--ignoring those spectator ions that really don't get involved.
What is the difference between a complete ionic equation and a net ionic equation?
A net ionic equation shows only the chemical species that are involved in a reaction, while a complete ionic equation also includes the spectator ions. We can find the net ionic equation for a given reaction using the following steps:
How to find the net ionic equation?
A net ionic equation shows only the chemical species that are involved in a reaction, while a complete ionic equation also includes the spectator ions. We can find the net ionic equation for a given reaction using the following steps: 1 Write the balanced molecular equation for the reaction, including the state of each substance. 2 Using your knowledge of solubility rules, strong acids, and strong bases, rewrite the molecular equation as a complete ionic equation that shows which compounds are dissociated into ions.#N#[I haven't learned about strong acids and bases yet!] 3 Identify and cancel out the spectator ions (the ions that appear on both sides of the equation).#N#[Want to double check your work?]
How does sodium interact with water?
Each chloride ion is interacting with multiple water molecules through the positive dipole of the water, and each sodium ion is interacting with water molecules through the negative dipole of the water.
What happens when ions are involved in a reaction?
When ions are involved in a reaction, the equation for the reaction can be written with various levels of detail. Depending on which part of the reaction you are interested in, ...
What are spectator ions?
If we take a closer look at our complete ionic equation, we see that and are present on both sides of the reaction arrow. Ionic species that remain unchanged like this during a reaction are called spectator ions. Since spectator ions appear on both sides of the equation, we can cancel them out, similar to how we can cancel out equal terms on either side of a math equation:
What is a balanced equation?
A molecular equation is sometimes simply called a balanced equation. In a molecular equation, any ionic compounds or acids are represented as neutral compounds using their chemical formulas. The state of each substance is indicated in parentheses after the formula.
Is sulfuric acid a base?
Sulfuric acid, , is a strong acid that completely dissociates into and ions in aqueous solution. Sodium hydroxide, , is a strong base that completely dissociates into and ions in aqueous solution. When and are combined, the products are water and aqueous sodium sulfate, . This reaction is represented by the molecular equation below.
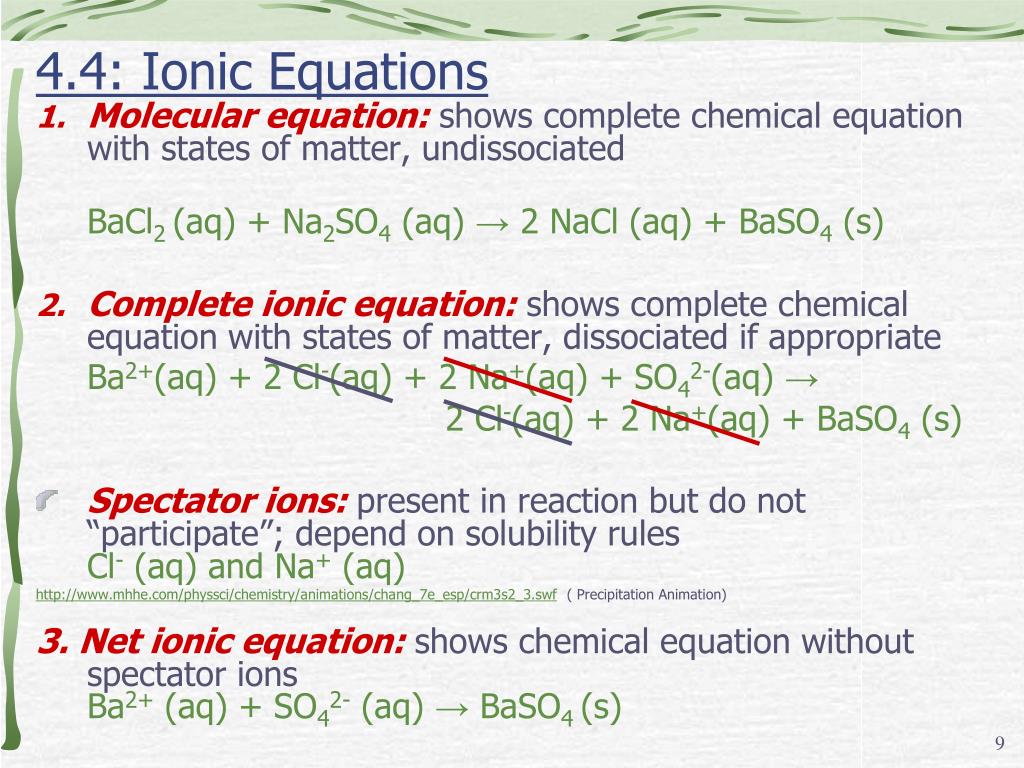
What is the equation for CaCl2 and Na2CO3?
CaCl2 (aq) + Na2CO3 (aq) → CaCO3 (s) + 2NaCl (aq), where (aq) means in solution and (s) means not in solution, but solid precipitate.
What is the complete ionic equation?
Your complete ionic equation includes all ions in solution, including spectator ions. Your net ionic equation leaves out spectator ions and focuses on what changes in the reaction.
What are Cl and Na?
Cl- and Na+ are on both sides of the equation in equal measure, and are generally referred to as “spectator ions”, in tha
What is the driving force of the reaction?
A valuable insight is that the driving force for the reaction is the precipitation of solid CaCO3, limestone, illustrating the principle Le Chatlier is famous for.
What is the standard method for dissociation?
The standard method is the calculate the equilibrium constant for dissociation of acid. We consider in the reaction H A → H + + A − in some media (can be a liquid solvent like water, benzene etc or in gas phase). The laws of thermodynamics tells
What does it mean when a strong acid and strong base react?
We know that strong acid and strong base mean strong electrolytes which are highly ionizable in solution. When they react to form a salt and water ,the reveraibility of reaction is least possible as the rate of forward reaction is higher due to high ionization of strong acid and base than the backward reaction which involves reaction between salt and water which is comparatively less ionic.
What happens after you determine the reaction (w/o) coefficients?
After you determine the reaction (w/o) coefficients, balance the elements and charge, if any.
How to go from a complete ionic equation to a net ionic equation?
to go from a complete ionic equation to a net ionic equation, all spectator ions are eliminated from the equation
Why is there no net ionic equation?
There is no net ionic equation because there is no reaction (all fours ions are exactly the same on each side, all of them are stricken out as being spectator ions). Often NR is used to signify no reaction. You will find additional NR examples at 13, 23, 28, and 44 in my problem sets. There are several examples at #44.
What is the mistake in ionic equations?
The mistake is to not show the proper coefficients when writing the complete ionic equation from the molecular equation. For example, when BaCl 2 ionizes, it forms one barium ion and two chloride ions in solution, not one of each.
Why do you start with names for complete molecular equations?
I started out with names for the complete molecular equations because your first answer in a given problem is often to translate the names into a complete molecular equation equation. This means that you must be able to (1) write correct chemical formulas from the names and (2) balance chemical equations.
Which side of the equation is reactant soluble?
The reactants are both soluble and ionize in solution, giving this on the left-hand side of the complete ionic equation:
Which ion is the spectator ion?
In both of the example equations above, the sodium ion and the chloride ion are the spectator ions. Here's why identifying the spectator ions is important: to go from a complete ionic equation to a net ionic equation, all spectator ions are eliminated from the equation.
Why do we have to know all the one offs?
You have to know them all (even the one-offs) because you never know what a particular teacher/textbook might use. Or, perhaps, a future lab partner who learned it one way, while you had learned it a different way.
What are the two most common forms of ionic equations?
The two most common forms of ionic equations are complete ionic equations and net ionic equations. The complete ionic equation indicates all of the dissociated ions in a chemical reaction. The net ionic equation cancels out ions that appear on both sides of the reaction arrow because they essentially don't participate in the reaction of interest.
What is an ionic equation?
Similar to a molecular equation, which expresses compounds as molecules, an ionic equation is a chemical equation in which the electrolytes in aqueous solution are expressed as dissociated ions. Usually, this is a salt dissolved in water, where the ionic species are followed by ...
Why are strong acids written as ions?
Strong acids, strong bases, and soluble ionic compounds (usually salts) exist as dissociated ions in aqueous solution, so they are written as ions in the ionic equation. Weak acids and bases and insoluble salts are usually written using their molecular formulas because only a small amount of them dissociates into ions.
How are ions stabilized in aqueous solutions?
The ions in aqueous solutions are stabilized by ion-dipole interactions with water molecules. However, an ionic equation may be written for any electrolyte that dissociates and reacts in a polar solvent. In a balanced ionic equation, the number and type of atoms are the same on both sides of the reaction arrow.
Step 1: Writing the Complete Balanced Molecular Equation
The first step of balancing ionic equations is balancing the molecular equation. Adjust the coefficients of the reaction to ensure that there is an equal number of each element on both sides of the reaction arrow. For example, {eq}HCl + Ba (OH)_2 \rightarrow BaCl_2 + H_2O {/eq} is balanced as {eq}2HCl + Ba (OH)_2 \rightarrow BaCl_2 + 2H_2O {/eq}.
Step 2: Writing the Complete Ionic Equation
Convert the complete balanced molecular equation (the result of step 1) into the complete ionic equation by separating any soluble ionic compounds into ionic form and leaving any other species (solids, gases, and liquids) as is. All ions should be labeled (aq).
Step 3: Writing the Net Ionic Equation
If an ion is soluble before and after the reaction, then it did not truly participate in the reaction. These ions are called spectator ions. When learning how to find net ionic equation spectator ions, simply look to see which ions are aqueous on both the reactant and product sides of the reaction.
What are the two types of ionic equations?
There are two types of ionic equations as complete ionic equation and net ionic equation. The complete ionic equation is a chemical equation that explains the chemical reaction, clearly indicating the ionic species present in a solution. An ionic species is either an anion (negatively charged species) or a cation (positively charged species).
What is the Difference Between Molecular Equation and Ionic Equation?
The key difference between molecular equation and ionic equation is that the molecular equation shows the reactants and products in molecular form, while the ionic equation only shows ionic species. Thus, the molecular equation is given in the molecular form, whereas the ionic equation is given in the ionic form. For example, let us look at the reaction between sodium chloride and silver nitrate, which gives a white precipitate known as silver chloride. Its molecular equation is NaCl + AgNO 3 ⟶ AgCl + NaNO 3 while the ionic equation is Na + + Cl – + Ag + + NO 3– ⟶ AgCl + Na + + NO 3–.
What is a Molecular Equation?
A molecular equation represents the reactants and products in molecular form. In contrast, an ionic equation gives only the ionic species involved in the chemical reaction. Therefore, in the molecular equation, we should not include any ionic species, only molecules. For example, the reaction between sodium chloride and silver nitrate gives a white precipitate known as silver chloride. The molecular equation for this reaction is as follows:
What is the molecular equation for sodium chloride and silver nitrate?
The molecular equation for this reaction is as follows: NaCl + AgNO 3 ⟶ AgCl + NaNO 3.
What are the different forms of chemical equations?
There are different forms of chemical equations, such as molecular equations and ionic equations. In this article, let’s examine the difference between molecular equation and ionic equation.
What is the reaction between two compounds called?
The compounds that undergo a certain chemical reaction is called a reactant, and what we get at the end is called the product. There are different forms of chemical equations, such as molecular equations and ionic equations.
What is the molecular equation of silver chloride?
Its molecular equation is NaCl + AgNO 3 ⟶ AgCl + NaNO 3 while the ionic equation is Na + + ...
Which equation shows the equation in which all the species of the reactants and the products are in dissociated form?
The net ionic equations shows the equation in which all the species of the reactants and the products are in dissociated form and do not show the spectator ions which are same in the reactants and the products.
How much HCI is in 10 ml of hydrochloric acid?
4. The 10 mL of hydrochloric acid contained 0.030 mol HCI. Perform stoichiometrie calculations to determine why the volume of the balloon with 0.15 g …
Molecular Equation
There are various ways to write out precipitation reactions. In the molecular equation, electrolytes are written as salts followed by ( aq) to indicate that the electrolytes are completely dissociated into their constituent ions; the ( aq) designation indicates that the ions are in aqueous solution.
Complete Ionic and Net Ionic Equations
Because the reactants and one of the products are strong electrolytes, it is possible to write them out in terms of their constituent ions. The resulting equation is known as the complete ionic equation, and it looks as follows:
Licenses and Attributions
The materials found on Course Hero are not endorsed, affiliated or sponsored by the authors of the above study guide