Reactions of Dehydration Synthesis and Hydrolysis
| Type of Organic Compound | Type of Bond Involved | Dehydration Synthesis | Hydrolysis |
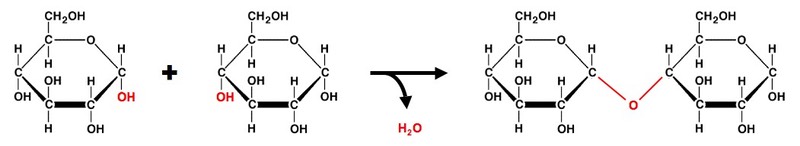
| Carbohydrates | Glycosidic bond | Two or more monosaccharides combined = . ... | Disaccharide/Polysaccharide + H 2 O = .. ... |
| Proteins | Peptide bond | Two or more amino acids =Dipeptide/Prote ... | Dipeptide/Protein + H 2 O = Two or more ... |
| Lipids | Ester bond | 3 Fatty acids + Glycerol = Lipid molecul ... | Lipid molecule + 3 H 2 O = 3 Fatty acids ... |
| Nucleic acids | Phosphodiester bond | 10 nucleotides = Nucleic acid + H 2 O | Nucleic acid + H 2 O = 10 nucleotides |
How do you reverse dehydration synthesis?
How do you check for dehydration?
- Gently pinch the skin on your arm or stomach with two fingers so that it makes a “tent” shape.
- Let the skin go.
- Check to see if the skin springs back to its normal position in one to three seconds.
- If the skin is slow to return to normal, you might be dehydrated.
What happens during a dehydration synthesis reaction?
In a dehydration synthesis reaction (Figure), the hydrogen of one monomer combines with the hydroxyl group of another monomer, releasing a molecule of water. At the same time, the monomers share electrons and form covalent bonds. As additional monomers join, this chain of repeating monomers forms a polymer.
What are the reactions of hydrolysis and condensation?
• Condensation reactions make chemical bonds whereas hydrolysis breaks chemical bonds. • Polymers are made by condensation reactions, and they are broken by hydrolysis reactions. • During condensation reactions, water molecule can be released. In the hydrolysis reactions, water molecule is incorporated into the molecule.
Is hydrolysis the same as digestion?
Therefore, the chemistry of digestion is simple because, in the case of all three major types of food, the same basic process ofhydrolysis is involved. The only difference lies in the types of enzymes required to promote the hydrolysis reactions for each type of food. All the digestive enzymes are proteins.

What are similarities between dehydration synthesis and hydrolysis?
There are two processes that play vital roles in biosynthesis. These are Hydrolysis and Dehydration Synthesis. Hydrolysis and Dehydration Synthesis both deal with water and other molecules, but in very different ways. Both have a reverse reaction in relation to each other and vice versa.
What is the relationship between hydrolysis and dehydration synthesis quizlet?
dehydration synthesis removes water while hydrolysis digests it.
What is the similarity and difference of hydration and hydrolysis reaction?
The difference between hydration and hydrolysis is that hydrolysis is the process of breaking of compounds using water, whereas hydration is defined as the electrophilic addition reaction, and there is no cleavage of the original molecule.
How are dehydration synthesis and hydrolysis opposites of each other be sure that your answer includes the mention of h2o quizlet?
Dehydration synthesis is essentially the opposite of hydrolysis. Dehydration is when you start off with 2 monomers and hydrolysis is when you start of with a polymer and water.
What is the difference between dehydration synthesis and hydrolysis?
Dehydration synthesis reactions build molecules up and generally require energy, while hydrolysis reactions break molecules down and generally release energy. Carbohydrates, proteins, and nucleic acids are built up and broken down via these types of reactions, although the monomers involved are different in each case.
Which of the following best summarizes the relationship between dehydration reactions and hydrolysis?
Which of the following best summarizes the relationship between dehydration reactions and hydrolysis? Hydrolysis creates monomers, and dehydration reactions break down polymers.
How are hydrolysis and condensation reactions related?
Hydrolysis reactions occur through the formation of smaller molecules from a larger reactant molecule. Therefore, condensation involves the formation of a new chemical bond while hydrolysis involves the breakdown of a chemical bond.
What is the difference between hydrolysis and hydrolysis?
Hydrolysis: Hydrolysis is a double decomposition reaction with water as one of the reactants. Hydration: Hydration is a chemical process in which water molecules combine with a substance.
What is the role of hydrolysis in the body?
The -H attaches to one monomer and the -OH goes off with another. Hydrolysis is an important part of how your body breaks food into its nutritious components. The food you eat enters your body in the form of polymers that are far too large to be used by your cells, so they must be broken down into smaller monomers.
What is the DNA that is created by dehydration?
Your DNA is built from just four kinds of monomers called nucleotides. Since all of us have the same monomers to build DNA from, it's the arrangement of the monomers that makes each of us unique.
What are the two ways the body works?
Your body is at work all the time. Keeping you alive and well is a big job! One way it does this is through two important reactions called dehydration and hydrolysis. Dehydrat ion reactions link monomers together into polymers by releasing water, and hydrolysis breaks polymers into monomers using a water molecule.
How to rehydrate yourself when dehydrated?
When you are dehydrated, you remedy this by hydrating - you add water to your body to rehydrate yourself. Hydrolysis, which is the reverse of a dehydration reaction, works the same way. In these reactions, polymers are broken apart by adding a water molecule and separating the polymer back into monomers.
How do enzymes help dehydration?
You digest them by hydrolysis, breaking them apart with water molecules. Enzymes Aid Both Reactions. Both dehydration reactions and hydrolysis need help, which they get from enzymes. These are specialized macromolecules that speed up chemical reactions in cells.
How are macromolecules made?
Most macromolecules are made by joining together small molecules called monomers. This literally means 'single unit' ('mono' means 'one'), and these are the single-unit molecules that combine to form polymers. What are polymers? Simply put, they are chains of monomers.
Why do we drink water when we are thirsty?
When you are thirsty, it's your body's way of telling you to drink some water because you are dehydrated. Just like a dehydration reaction, you've lost water. Dehydration reactions are named as such because as the monomers are linked together, a molecule of water is released.
What are some examples of dehydration synthesis reactions?
1. Biochemical reactions are one of the best examples of dehydration synthesis reactions. Amino acids are building blocks of proteins as they polymerize to form peptides and polypeptides. Amino acids have two functional groups; amino -NH 2 and carboxylic group (-COOH). They react to form an amide linkage (-CO-NH-) with the elimination of water molecule.
What is the process of dehydration?
Dehydration Synthesis and Hydrolysis. Dehydration synthesis involves the formation of new chemical bonds between two molecules which leads to the formation of new compounds. A reaction occurs with the loss of water molecule at each step. The loss of water molecule can occur due to reaction between two functional groups like –OH, -NH 2 or –COOH.
What is the formation of peptides from amino acids?
For example formation of the peptide from amino acids is a dehydration synthesis reaction. It is a peptide bond formation reaction which occurs between two amino acid molecules. The amino group of one molecule and carboxyl group of another molecule condenses with the elimination of water molecule and form an amide linkage in dipeptide.
What is an example of an elimination reaction?
Unlike substitution reactions, elimination reactions involve the elimination of some atoms in the form of good leaving groups such as water, halogen etc. For example; elimination of water molecule from alcohol forms alkene. It is an example of a dehydration reaction.
What is the name of the reaction that involves the elimination of water molecules?
Dehydration Synthesis Reaction. Dehydration synthesis can be defined as the synthesis reactions which involve the formation of a new compound with the elimination of water molecule. Since these reactions result in the formation of a new compound with a large structure, therefore, they are called as synthesis reactions.
What is the term for the elimination of water?
This is because the term dehydration is used for ‘losing water’ and synthesis represents the formation of the new substance, therefore, dehydration synthesis is the elimination of water with the formation of new compounds. Definitely combination of two molecules will form a large compound and water molecule will eliminate ...
How many steps are involved in dehydration synthesis?
So we can say that a dehydration synthesis always involves two steps. Formation of a new product. Loss of water molecule.
What is the difference between dehydration and hydrolysis?
The main difference between dehydration synthesis and hydrolysis is that dehydration synthesis results in the formation of a large molecule out of smaller molecules whereas hydrolysis results in the formation of smaller molecules out of a large molecule.
How is dehydration used in chemical synthesis?
In chemical synthesis processes, dehydration synthesis is used to obtain larger molecules through releasing water molecules. For example, the reaction between an alcohol and a carboxylic acid may produce an ester, releasing a water molecule as the byproduct. Here, the –OH group of the carboxylic acid is released and the –H atom bonded to ...
What is hydrolysis reaction?
Hydrolysis is cleavage of a chemical bond in the presence of water. Here, water act as a reactant that is involved in the reaction process. In hydrolysis reactions, a large molecule is always broken down into smaller molecules. Therefore, the reactants always include a complex molecule, unlike products. In these reactions, a water molecule is added ...
What is the term for the formation of a larger molecule with the release of water molecules?
Definition. Dehydration: Dehydration synthesis is the formation of a larger molecule with the release of water molecules. Hydrolysis: Hydrolysis is cleavage of a chemical bond in the presence of water.
What is the name of the reaction that occurs between a hydroxyl group and a proton?
These dehydration synthesis reactions can also be named as condensation reactions since condensation refers to the formation of water molecules. Therefore, dehydration synthesis occurs between molecules having a hydroxyl (–OH) group and a proton that are available to be released.
What are the two types of chemical reactions?
Chemical reactions can be categorized into different groups according to the properties of those reactions. Dehydration synthesis and hydrolysis are such chemical reactions. These reactions are categorized according to their mechanism. Both these reactions involve either synthesis or consumption of water molecules. The main difference between dehydration synthesis and hydrolysis is that dehydration synthesis results in the formation of a large molecule out of smaller molecules whereas hydrolysis results in the formation of smaller molecules out of a large molecule.
How are peptide bonds formed?
Glycosidic bonds are formed as a result of the reaction between two monosaccharides releasing a water molecule whereas peptide bonds are formed as a result of the reaction between two amino acids releasing a water molecule. Therefore, these are condensation or dehydration synthesis reactions.