Working scientifically
| Element | Melting point |
| Lithium, Li | 180°C |
| Sodium, Na | 98°C |
| Potassium, K | 63°C |
| Rubidium, Rb | 39°C |
How do you classify elements in the periodic table?
What are Orbitals?
- The center of the atom is called the nucleus.
- Electrons are found in areas called shells. A shell is sometimes called an energy level.
- Shells are areas that surround the center of an atom.
What does the periodic table list elements according to?
The Periodic Table
- The Periodic Table. The periodic table shows all the elements and their physical properties; it is arranged based on atomic numbers and electron configurations.
- Molecules. Molecules are electrically neutral compounds made of multiple atoms bound together by chemical bonds.
- Ions. ...
What element is not listed on the periodic table?
The first chemical element is Actinium and the last is Zirconium. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor (s), non-metal (s), inert noble gas (ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals.
How are the elements grouped in the periodic table?
- Group 1 contains elements containing only 1 valence electron in their atoms, these are called alkali metals such as lithium, sodium, potassium, etc. ...
- Group 2 contains alkaline earth metals like beryllium, magnesium, calcium, and so on have 2 valence electrons. ...
- Group 17 contains halogens like fluorine, chlorine, bromine, etc. ...

How does the modern periodic table classify elements?
A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 2). Each box represents an element and contains its atomic number, symbol, average atomic mass, and (sometimes) name.
What are the classification of periodic table?
Ans: Based on the properties, elements are classified into \(3\) types. They are metals, non-metals and metalloids.
What is an element and how are elements classified?
What is an Element? The chemical element read as follows: a species of atoms all atoms with the same number of protons in the atomic nucleus. A pure chemical substance composed of atoms with the same number of protons in the atomic nucleus.
What are the 3 classification of elements?
Three classes of elements are metals, nonmetals, and metalloids. Across a period, the properties of elements become less metallic and more nonmetallic.
Which elements belong to group 15 of the modern periodic table?
In the modern periodic table of elements, the following fall under group 15: Nitrogen (N) Phosphorus (P) Arsenic (As) Antimony (Sb) Bismuth (Bi) Th...
What trends in electronegativity can be seen in the modern periodic table of elements?
In the modern periodic table, the electronegativity of elements increases across a period (row) and decreases down a group (column). Therefore, the...
How are the elements classified into different blocks in the modern periodic table?
The modern periodic table of elements can be broken down into 4 blocks – the s-block, the p-block, the d-block, and the f-block. This classificatio...
What are the f-block elements?
The f-block elements are the elements of the modern periodic table whose valence electrons lie in f-orbitals. These elements can be broadly classif...
What are the trends in the atomic/ionic radii of elements in the modern periodic table?
The atomic/ionic radii of elements increases while traversing down a group in the modern periodic table due to the addition of new electron shells....
What is the modern periodic table?
The periodic table commonly called as the periodic table of elements is the representation of chemical elements in the increasing order atomic numb...
How are elements classified in the modern periodic table?
Elements are classified into terms of their increasing atomic number in the modern periodic table.
How many rows and columns are there in the modern periodic table
The current periodic table has been divided into seven periods (also known as horizontal rows) and eighteen groups (also known as vertical columns)...
How many elements constitute the first period?
There are only two elements in the first period. This is the shortest period.
How many man made elements are there in the periodic table?
There were a total of 118 elements recognized by the International Union of Pure and Applied Chemistry. The first 94 elements are found naturally o...
Why do atomic radii decrease?
The atomic radii of the elements generally decrease across periods due to the increase in electronegativity and the increase in the effective nuclear charge acting on the outermost shells.
What are the f block elements?
The f-block elements are the elements of the modern periodic table whose valence electrons lie in f-orbitals. These elements can be broadly classified into two categories. The lanthanides – elements whose valence electrons lie in the 4f orbital. The actinides – elements whose valence electrons lie in the 5f orbital.
How many columns are there in the periodic table?
There are eighteen vertical columns known as groups in the modern periodic table which are arranged from left to right and seven horizontal rows which are known as periods. The elements of this group form salts. They are noble gases and under normal conditions they are inert.
How many elements are in a vertical column?
Earlier scientists assumed that the properties of elements are periodic functions of their atomic masses. On the basis of this assumption, Mendeleev placed 63 elements in a vertical column called groups and in horizontal rows called periods.
What is the long group of elements?
Period four and five have eighteen elements and are known as the long group. In the modern periodic table, group number 3 of period six contains the lanthanide series which are the rare earth elements. We have radioactive elements (actinides) present in group 3 of period seven.
Which element has the lowest electronegativity?
In the modern periodic table, the electronegativity of elements increases across a period (row) and decreases down a group (column). Therefore, the bottom-left most element (francium) is predicted to have the lowest electronegativity and the top-right most element (fluorine) is predicted to have the highest electronegativity.
What are the elements of group 18?
The elements of group 1, 2, 13, 14, 15, 16, and 17 are known as the main group elements or normal elements. The elements of groups 3, 4, 5, 6, 7, 8, 9, 11 and 12 are known as the transition elements. Group 18 is called noble gases or inert gases. Their outermost shell is completely filled. Due to this stable electronic configuration, they generally don’t react with the other elements.
Why do we need to classify elements and what was the need for classification of elements?
The periodic table commonly called as periodic table of elements is representation of chemical elements in increasing order atomic number of elements, chemical properties, as well as order of their electronic configuration. The table comprises of 118 total elements
Modern Periodic Table
Periodic properties of elements are determined by periodic functions of atomic numbers, according to current periodic law.
What is the need for classification of elements? Why do we classify elements Class 10?
As new elements were discovered, this becomes important to schedule as well as classify all existing elements. Physical as well as chemical properties, as well as atomic mass, were examined, therefore. Various researchers tried to describe elements based on chemical as well as physical characteristics.
How are elements classified in the modern periodic table classification?
Periodic classification of elements class 11 is a method for classifying elements based on their features, in which we keep elements that are similar in one group and the rest of the elements in the other.
Classification of elements into periods in the periodic table
The periodic table's horizontal row is called a period. The periodic table is divided into seven periods, each starting on the far left. When a new primary energy level begins to fill with electrons, a new period begins. Period 1 only includes two elements (hydrogen and helium), but periods 2 and 3 each have eight.
Classification of elements into groups in the periodic table
Modern periodic tables have vertical columns called groups. In periodic table, there are 18 groups. one through eighteen groups is numbered. The elements in each group have same outer shell electronic configuration.
Points to Remember
Periods (rows) and groups (columns) make up the modern Periodic Table (columns). There are 18 groups, each with a different number of periods ranging from one to seven.
What is the modern periodic table based on?
The modern periodic law was accepted widely, and it is the reason the modern periodic table that we use today is based on the modern periodic law.
What are the properties of elements?
According to modern periodic law, the properties of elements are periodic functions of atomic numbers and not atomic masses which means the properties of elements occur periodically or repeat themselves periodically when elements are arranged in an order of increasing atomic numbers.
What is the lower row of the f block called?
This row or series of elements is called the Actinide series as its first element is Actinium with atomic number 89.
How many elements were in Dobereiner's triad?
Dobereiner's Triads: Around 33 elements were known when Dobereiner gave his theory for the classification of elements. He arranged the elements in groups of three or as triads in such a way that the atomic weight of the element at the middle was the arithmetic mean of the other two elements. However, his theory was not accepted as only 9 elements ...
What is periodic classification?
In the classification of elements, the elements are arranged in groups which are known as periods, so this classification system is known as the periodic classification of elements.
What does Mendeleev say about empty spaces?
Mendeleev suggested that these empty spaces indicate that there are elements that are yet to be discovered, so, he said that these empty spaces would be occupied by the elements that are not discovered yet. He even predicted the properties of these elements in advance based on the arrangement of elements in his table.
How many elements are in the periodic table?
The modern periodic table contains 118 elements. It is divided into four blocks that include S block, P block, D block and F block. These blocks contain seven horizontal rows of elements, which are known as periods and 18 vertical columns of elements that are known as groups. Group 1 and 2 are placed in the S block, 13 to 18 groups are placed in the P block and the remaining groups from 3 to 12 are placed in the d block of the modern periodic table.
What is the Moseley periodic law?
Moseley's Periodic Law. Henry Moseley showed that the chemical and physical properties of elements is determined by the atomic number and not by the atomic mass. He restated the periodic law as-. 'Physical and chemical properties of an element are a periodic function of its atomic number.'. The Moseley's periodic law is also known as ...
What are the elements that exhibit the properties of metals and non-metals called?
These elements exhibit the properties of metals and non-metals, thus, they are called metalloids. Noble gases: The extreme right side of the periodic table is occupied by gases. They are placed in the 18th group and have completely filled valence shells. These gases are non-reactive and are called the inert or noble gases.
What did Johann Döbereiner do?
He grouped the elements into gases, non-metals, metals and earthly elements. After several decades, in 1829 Johann Döbereiner made an attempt to group elements. Based on the similarity in chemical properties, he grouped elements into triads.
How many rows are there in the periodic table?
Following are some of the main features of modern periodic table. Elements are grouped in ascending order of their respective atomic number. There are seven horizontal rows called periods and eighteen vertical columns called groups.
Where are transition metals placed on the periodic table?
Some of the transition metals are placed separately in two rows at the bottom of the periodic table. These are known as Lanthanides and Actinides. Metalloids and non-metals: Metalloids generally appear in a diagonal line at the right side of the periodic table.
Which group of elements is the left side of the periodic table?
Alkali and Alkaline Earth metals: The first two groups on the left side of the periodic table consists of highly reactive elements (except hydrogen). The first group elements contain one electron in the valence shell while the second group elements contain two electrons in their valence shell. Transition metals: These elements occupy the centre ...
When did Mendeleev publish his paper?
It was only in 1869 that Mendeleev published his paper in the Journal of Russian Chemical Society where he classified the elements based on their atomic masses and arranged them into horizontal rows called periods and vertical columns called groups.
Why is periodic classification important?
Significance of the Periodic Classification of Elements 1 Makes the study of elements easy: Classification of elements in groups provide us with a fixed pattern in which the elements change their properties periodically. The periodic table made the study of the physical and chemical properties of elements simple and organized. We can now just go to the group and see the properties of the elements of the periodic table or predict the properties of an element if we know the characteristics of other elements present in the same group. 2 Helps in discovering new elements: Although there are so many elements that are already discovered, there are chances that new elements can be discovered. Scientists can take the help of a periodic table and know the trending characteristics based on the properties of elements and hence can identify the new elements with the existing ones. Besides, researchers are continuously striving to discover new elements and explore their properties.
How are the elements arranged in the long form periodic table?
In the long form periodic table the elements are arranged in the order of their atomic numbers. Atomic number of an element is equal to the number of protons inside the nucleus of its atom.
What are the main elements in the periodic table?
In chemistry and atomic physics the main group is the group of elements (sometimes referred to as representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen , oxygen, and fluorine as arranged in the periodic table of elements.
How many triads did Dobereiner find?
Only three triads could Dobereiner find; I.e a total of only 9 elements. The total number of elements, however, was more than that of those included in the Triad of Dobereiner. Thus, most of the elements known at that time could not be classified by the Dobereiner’s.
What are the elements of Group 3 to 12 called?
The elements of Groups 3 to 12 are called transition elements . Elements with atomic number 58 to 71 (Ce to Lu) occurring after lanthanum (La) are called lanthanides. Elements with atomic numbers 90 to 103 (Th to Lw) are called actinides. These elements are called f-block elements and also as inner transition elements.
Why did scientists start thinking about a method to study elements?
In order to ease the work, scientists started thinking about a method so that the study of elements can be simplified. They decided to organize the elements in a periodic table according to the information available about the elements and various characteristics shown by them.
How many periods are there in the periodic table?
There are seven horizontal rows called periods in the long form periodic table. Thus, there are seven periods in the long form periodic table. The elements of Groups 1, 2 and 13 to 17 are called the main group elements. These are also called typical or representative or normal elements. The elements of Groups 3 to 12 are called transition elements.
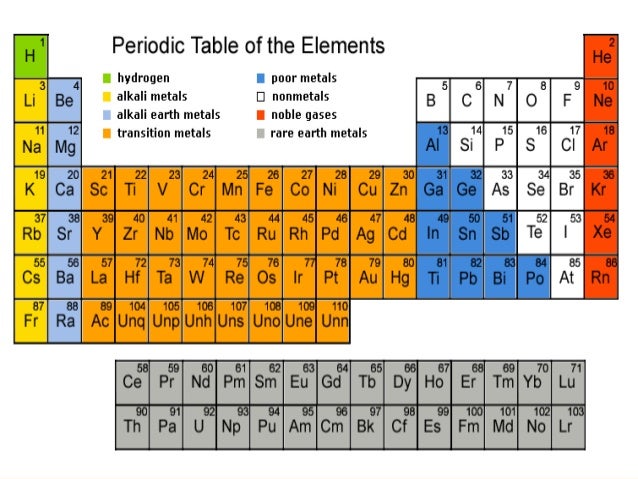
How Are Elements in The Modern Periodic Table Classified?
- The method by which elements are classified based on their characteristics is a periodic classification of elements i.e. we hold the elements that are similar in one group and the rest of the elements in the other group. In the periodic table, several empty spaces have been left to position the elements that will be found in the future without disr...
What Is The Need For The Classification of elements?
- It is difficult to individually study every element and to know its properties and uses. Therefore, based on their similarities in properties, they were classified. In the Periodic Table, the structured classification of the elements has helped chemists to study and understand the properties of the elements and their compounds more systematically and orderly.
Advantages of Classification of Elements in The Modern Periodic Table
- If the position of an element is known, It becomes easier to remember the properties of an element.
- Unlike Mendeleev’s periodic table, positions of isotopes are taken care of within one element itself.
- The metals, non-metals, transition metals, gases are separately placed in a particular locatio…
- If the position of an element is known, It becomes easier to remember the properties of an element.
- Unlike Mendeleev’s periodic table, positions of isotopes are taken care of within one element itself.
- The metals, non-metals, transition metals, gases are separately placed in a particular location with a specific identity in the modern periodic table.
- The classification of elements is colour on the atomic number, which is a more basic property.
The Constituents of The Modern Periodic Table
- The periodic table classification has been done with mainly four types of elements. The elements present in the modern periodic table are : 1. The noble gases: a group of rare gases that consist of helium, radon, neon, argon, krypton, and xenon 2. The main transition element: Electrons that can engage in chemical bond formation in two shells instead of just one shell. 3. The representative …