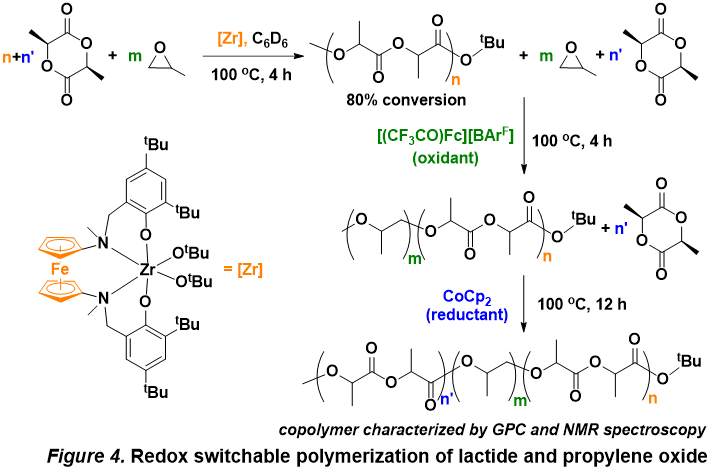
Esters are easily made in the laboratory from their corresponding carboxylic acid and a condensation reaction with an alcohol. This reaction is condensed and catalyzed with concentrated (18M) sulfuric acid.
How do you synthesize esters?
This lab contained the synthesis of an ester by allowing different carboxylic acids to react with alcohols, using sulphuric acid as a catalyst, to create esters and allow the student to identify them by their distinct smell. The smell was distinguished by the different compounds that were created in the dehydration synthesis reaction.
What is ester preparation?
Ester Preparation Lab. An ester is an organic compound which is created from a reaction between an acid and an alcohol, usually with the loss of water. Many esters contain veer distinct odors, which has led to them being used for artificial flavoring and fragrances. Esters can be synthesized artificially in labs by combining alcohols and acids...
What is the purpose of the ester synthesis lab?
Purpose: The purpose of this lab was to observe the synthesis of esters and identify the odor of each and then to write chemical equations of each ester synthesized.
What is an esterification reaction?
An esterification reaction is a condensation reaction in which a carboxylic acid and an alcohol combine to produce an ester and water (Van Kessel et al, 2003). Esters are organic chemicals that contain an ester linkage, , and are formed when an alcohol and an organic acid react together.
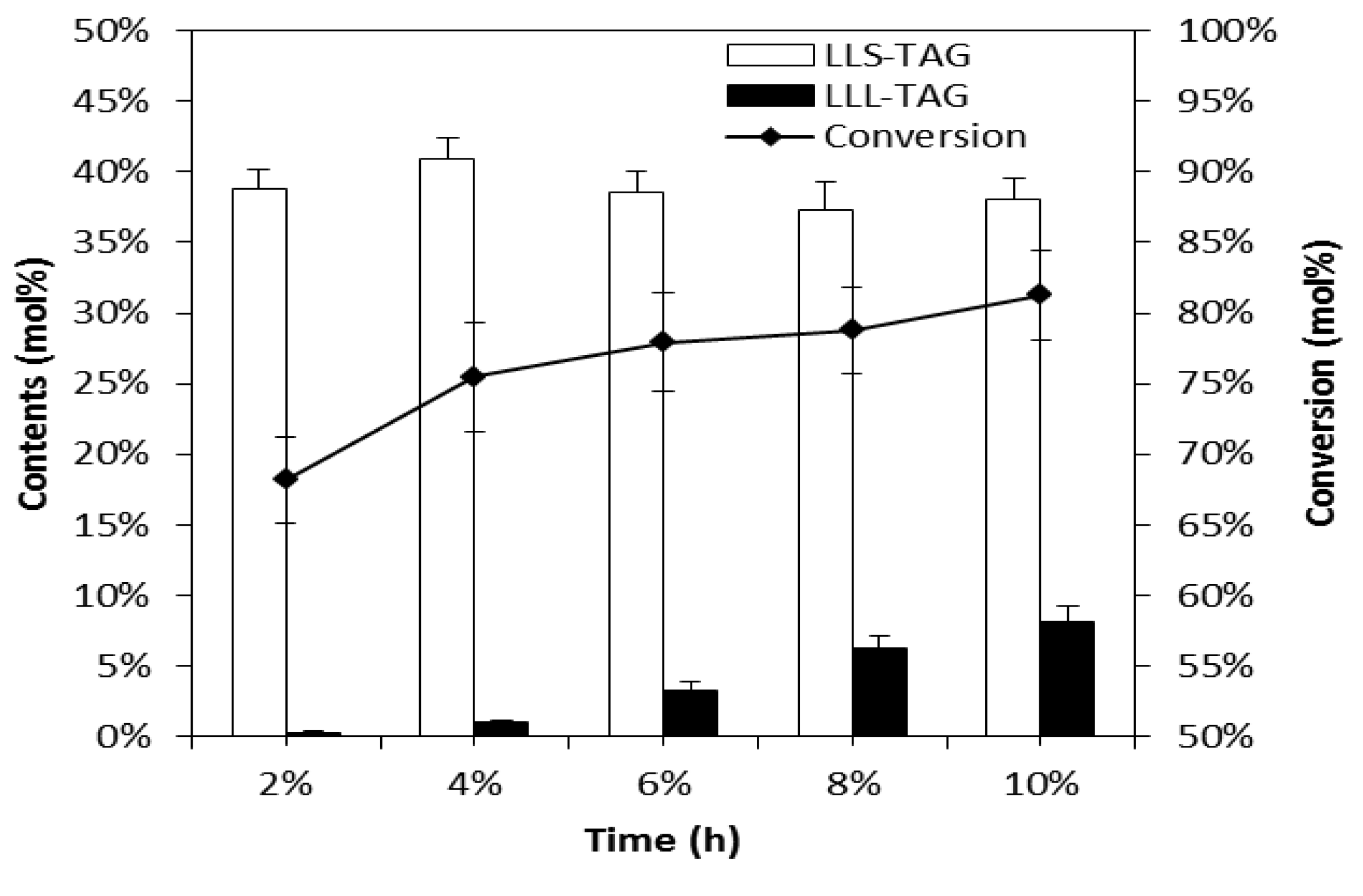
How can esters be synthesized?
Esters are commonly synthesized from carboxylic acids by reaction of the acid with an excess of alcohol containing a catalytic amount of a mineral acid. In cases where practical considerations dictate it, the acid can be converted to an acyl halide (usually the chloride) and then condensed with the appropriate alcohol.
How are esters prepared in a school lab?
ProcedureAdd 10 drops of ethanoic acid (or propanoic acid) to the sulfuric acid in the specimen tube.Add 10 drops of ethanol (or other alcohol) to the mixture.Put about 10 cm3 of water into the 100 cm3 beaker. ... Heat the beaker gently on a tripod and gauze until the water begins to boil, then stop heating.More items...
What does it mean to synthesize an ester?
Reaction type: Nucleophilic Acyl Substitution. Summary. This reaction is also known as the Fischer esterification. Esters are obtained by refluxing the parent carboxylic acid with the appropraite alcohol with an acid catalyst.
What are esters and how are they formed?
Esters are formed as a result of chemical reaction called esterification. Reaction: When primary alcohol reacted with a carboxylic acid in the presence of sulphuric acid ester compound is formed. RCOOH+ROH⟶RCOOR+H2O. Uses of esters. (i) Esters have pleasant smell.
What is the purpose of ester synthesis lab?
Purpose: The purpose of this lab was to observe the synthesis of esters and identify the odor of each and then to write chemical equations of each ester synthesized. colorless liquid with a pungent, putrid odor.
What is the process of esterification?
Esterification is the process of combining an organic acid (RCOOH) with an alcohol (ROH) to form an ester (RCOOR) and water; or a chemical reaction resulting in the formation of at least one ester product.
Why are esters prepared using a water bath?
The purpose of water bath during the preparation of esters is to purify the ester by separating the alcohol and acid which dissolves in water can be tucked safely under the ester layer.
Why is an ester poured into cold water?
The reaction mixture is poured over ice to precipitate the crude product. Ice is used because the reaction of concentrated acids, especially concentrated sulfuric acid, with water is very exothermic. This would lead to the hydrolysis of the ester function during this step.
What is the purpose of esterification?
Esterification can increase the volatility of fatty acids, reduce dimerization in the vapor phase, and reduce adhesion. Esterification improves the peak configuration, the separation, and sample detectability. The methyl, ethyl, propyl, iso-propyl, n-butyl, and iso-butyl esters of fatty acids are recommended.
How are esters formed where they occur in nature give one example?
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are ubiquitous. Most naturally occurring fats and oils are the fatty acid esters of glycerol.
Which reactions can be used to prepare an ester?
Esters are made by the reaction of a carboxylic acid with an alcohol, a process that is called esterification.
How will you convert an acid into an ester without using an alcohol?
Solution : Using -diazomethane. Acid reacts with diazomethane `(CH_2 ~N_2)` to form an ester.
Which reactions can be used to prepare an ester?
Esters are formed by reaction of acids with alcohols: Organic acid + Alcohol → Ester + Water. e.g.
Why is a hot water bath used in the preparation of esters?
Explanation: The water bath will help the ester to get separated from the alcohol and the acids so that we can obtain the pure ester at the end of the process. The acids and alcohol will get dissolved in water and they form a thin layer in the beaker where ester is stored.
How long does it take to make an ester?
A very simple method of making an ester You can mix equal volumes of small quantities of a carboxylic acid and an alcohol with an even smaller volume of concentrated sulfuric acid. The mixture is gently warmed in beaker of warm water for 5-10 minutes.
Where are esters found in everyday life?
Esters that have fragrant odours are used as a constituent of perfumes, essential oils, food flavourings, cosmetics, etc. It is used as an organic solvent. Natural esters are found in pheromones. Naturally occurring fats and oils are fatty acid esters of glycerol.