
How do I purify proteins secreted from cells in cell culture media?
For proteins that are secreted into the cell culture medium, remove any cells from the medium prior to purification. For more information, see Technical Manual #TM060. More information and detailed protocols for use of the MagneHis™ System are available in Technical Manual #TM060.
How do you isolate proteins from cells?
In order to extract the protein from the cells where it is present, it is necessary to isolate the cells by centrifugation. In particular, centrifugation using media with different densities may be useful to isolate proteins expressed in specific cells.
Why do scientists need to isolate and purify proteins of interest?
These techniques require scientists to isolate and purify proteins of interest so that their conformations and substrate specificities can be studied. Also requiring study are the reactions with other ligands (a protein that attaches to a receptor protein) and specific enzyme activities.
What are the first proteins to be purified?
The first proteins to be purified are water-soluble proteins. Purification of integral membrane proteins requires disruption of the cell membrane in order to isolate any one particular protein from others that are in the same membrane compartment.
How do you separate and purify proteins?
The methods used in protein purification, can roughly be divided into analytical and preparative methods....Many different chromatographic methods exist:Size Exclusion Chromatography: ... Ion Exchange Chromatography: ... Affinity Chromatography: ... Metal Binding: ... Immunoaffinity Chromatography: ... HPLC:
How are proteins purified?
In bulk protein purification, a common first step to isolate proteins is precipitation with ammonium sulfate (NH4)2SO4. This is performed by adding increasing amounts of ammonium sulfate and collecting the different fractions of precipitated protein. Subsequently, ammonium sulfate can be removed using dialysis.
How is protein extracted from cells?
Proteins may thereby be extracted from particular cell compartments via multiple rounds of centrifugation. Additionally, nucleic acids can be chemically precipitated out of the initial tissue slurry and removed by centrifugation. A similar technique can be used to precipitate proteins out of the supernatant.
How do you extract protein isolate?
Protein isolate made from local legumes can be made by extraction/acid hydrolysis and centrifugation methods, to supplement proteins with other components. Protein separation uses solvent acids to deposition proteins at isoelectric pH.
How can proteins be separated?
High-performance liquid chromatography (HPLC) can be used to separate and to purify proteins/peptides based on size, charge or overall hydrophobicity. Thin-layer chromatography (TLC) can also be used to separate out peptides (e.g., derived from proteolytic digestion of a protein) based on similar properties.
Why do we isolate proteins?
In order to be able to assign a particular function to a particular protein, it is usually necessary to isolate it so that it can be studied in the absence of other proteins.
What is isolation and purification?
Definition. Isolation, separation and purification refer to techniques used to isolate, concentrate or purify cells, viruses, cell fractions, organelles or biological macromolecules (e.g. proteins, protein complexes, chromatin, nucleic acids, carbohydrates or lipids) for subsequent analysis.
Which of the following is not the method of isolation and purification of protein?
Affinity chromatography Was this answer helpful?
How is protein isolate made?
Whey protein isolate is actually a by-product of cheesemaking. Whey protein begins its life as milk (usually cow's milk). Enzymes, heat or other catalysts are added to the milk to curdle it, which forces the liquids and the solids to separate.
What is meant by protein isolate?
Protein isolates are refined form of protein containing the greater amount of protein with greater digestibility.
What is isolate?
1 : to set apart from others also : quarantine. 2 : to select from among others especially : to separate from another substance so as to obtain pure or in a free state.
What is the first protein to be purified?
The first proteins to be purified are water-soluble proteins. Purification of integral membrane proteins requires disruption of the cell membrane in order to isolate any one particular protein from others that are in the same membrane compartment.
What is the purpose of protein purification?
Protein purification. Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein ...
Why is ion exchange chromatography important?
Because of the nature of the separating mechanism, pH, buffer type, buffer concentration, and temperature all play important roles in controlling the separation. Ion exchange chromatography is a very powerful tool for use in protein purification and is frequently used in both analytical and preparative separations.
How is chromatography used to separate proteins?
Chromatography can be used to separate protein in solution or denaturing conditions by using porous gels. This technique is known as size exclusion chromatography. The principle is that smaller molecules have to traverse a larger volume in a porous matrix. Consequentially, proteins of a certain range in size will require a variable volume of eluent (solvent) before being collected at the other end of the column of gel.
What happens when a sample is centrifuged?
If samples are centrifuged long enough, the particles in the vessel will reach equilibrium wherein the particles accumulate specifically at a point in the vessel where their buoyant density is balanced with centrifugal force. Such an "equilibrium" centrifugation can allow extensive purification of a given particle.
What is the first step of the purification process?
If the protein of interest is not secreted by the organism into the surrounding solution, the first step of each purification process is the disruption of the cells containing the protein.
Why is protein purification important?
Protein purification is vital for the specification of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Separation of one protein from all others is typically ...
1. Isolation of proteins (including enzymes)
Most proteins are soluble in water, dilute salts, dilute acids or alkaline solutions, and a few proteins linked to lipids are soluble in organic solvents such as ethanol, acetone, butanol, etc. , You can take different solvents to extract and purify proteins and enzymes separately.
1. Concentration of Samples
Biomacromolecules become very dilute due to column purification during the preparation process. In order to retain and identify the target, they often need to be concentrated. Commonly used concentration methods:
2. Monotonous
Products prepared from biological macromolecules are often monotonous to avoid deterioration and easy to retain. The most commonly used method is freezing and harmonious vacuum monotony. Vacuum monotony is suitable for the single harmonious preservation of substances that are not resistant to high temperature and easy to oxidize.
3. Storage
The consolidation of biological macromolecules has a great relationship with the retention method. Monotonous products are usually more consolidated, and their activity can be changed without obvious changes in days or even years at low temperatures. It is easy to plead for storage.
What is the most common tag used in protein purification?
The most commonly used tag is the polyhistidine tag (Yip et al. 1989). Protein purification using the polyhistidine tag relies on the affinity of histidine residues for immobilized metal such as nickel (Yip et al. 1989; Hutchens and Yip, 1990). This affinity interaction is believed to be a result of coordination of a nitrogen on the imidazole moiety of polyhistidine with a vacant coordination site on the metal. The metal is immobilized to a support through complex formation with a chelate that is covalently attached to the support.
How long does it take to mix protein with resin?
In batch mode, the protein of interest is bound to the resin by mixing lysate with the resin for approximately 30 minutes at a temperature range of 4–22°C. Once bound with protein, the resin is allowed to settle to the bottom of the container, and the spent lysate is removed.
What is the affinity tag for glutathione-S-transferase?
The use of the affinity tag glutathione-S-transferase (GST) is based on the strong affinity of GST for immobilized glutathione-covered matrices (Smith and Johnson, 1988). Glutathione-S-transferases are a family of multifunctional cytosolic proteins that are present in eukaryotic organisms (Mannervik and Danielson, 1988; Armstrong, 1997). GST isoforms are not normally found in bacteria; thus endogenous bacterial proteins don’t compete with the GST-fusion proteins for binding to the purification resin. The 26kDa GST affinity tag enhances the solubility of many eukaryotic proteins expressed in bacteria.
What is the best material for affinity purification?
The two most common support materials for resin-based, affinity-tagged protein purification are agarose and silica gel. As a chromatographic support, silica is advantageous because it has a rigid mechanical structure that is not vulnerable to swelling and can withstand large changes in pressure and flow rate without disintegrating or deforming. Silica is available in a wide range of pore and particle sizes including macroporous silica , which provides a higher capacity for large biomolecules such as proteins. However, two of the drawbacks of silica as a solid support for affinity purification are the limited reagent chemistry that is available and the relatively low efficiency of surface modification.
What is the name of the enzyme used in cell lysis?
Cell lysis can be accomplished a number of ways, including nonenzymatic methods (e.g., sonication or French press) or use of hydrolytic enzymes such as lysozyme or a detergent reagent such as FastBreak™ Cell Lysis Reagent. Because purification of native proteins can be challenging, affinity purification tags are often fused to a recombinant protein ...
What are the steps of purification?
There are four basic steps of protein purification: 1) cell lysis, 2) protein binding to a matrix, 3) washing and 4) elution. Cell lysis can be accomplished a number of ways, including nonenzymatic methods (e.g., sonication or French press) ...
What is immobilized protein used for?
Immobilized proteins also can be used in protein pull-down assays to isolate protein binding partners in vivo (mammalian cells) or in vitro. Other downstream applications such as mass spectrometry do not require protein immobilization to identify protein partners and individual components of protein complexes.
What is the first step in purifying intracellular proteins?
The first step in purifying intracellular (inside the cell) proteins is the preparation of a crude extract. The extract will contain a complex mixture of all the proteins from the cell cytoplasm, and some additional macromolecules, cofactors, and nutrients. This crude extract may be used for some applications in biotechnology.
What is the purity of protein?
The degree of protein purity required depends on the intended end use of the protein. For some applications, a crude extract is sufficient. Other uses, such as in foods and pharmaceuticals, a high level of purity is required. Several techniques for protein purification are used to reach a required purity level.
How to purify heat resistant proteins?
An easy approach to purifying a heat-resistant protein is to denature the other proteins in the mixture by heating, then cooling the solution (thus allowing the thermostable enzyme to reform or redissolve, if necessary). The denatured proteins can then be removed by centrifugation.
How to purify protein from crude extract?
Employ Precipitation. In the past, a common second step to purifying a protein from a crude extract was by precipitation in a solution with high osmotic strength (i.e. salt solutions). Protein precipitation is usually done using ammonium sulfate as the salt.
How to remove nucleic acids from crude extract?
Nucleic acids in the crude extract can be removed by precipitating aggregates formed with streptomycin sulfate or protamine sulfate. Salt precipitation does not usually lead to a highly purified protein but can assist in eliminating some unwanted proteins in a mixture, and by concentrating the sample.
What is ion exchange chromatography?
Ion-exchange chromatography refers to the separation of proteins based on charge. Columns can either be prepared for anion exchange or cation exchange. Anion exchange columns contain a stationary phase with a positive charge that attracts negatively charged proteins.
What is immunoblotting in chemistry?
Immunoblotting is a protein visualization technique applied in combination with affinity chromatography. Antibodies for a specific protein are used as ligands on an affinity chromatography column. The target protein is retained on the column, then removed by rinsing the column with a salt solution or other agents.
Overview
Preliminary steps
If the protein of interest is not secreted by the organism into the surrounding solution, the first step of each purification process is the disruption of the cells containing the protein. Depending on how fragile the protein is and how stable the cells are, one could, for instance, use one of the following methods: i) repeated freezing and thawing, ii) sonication, iii) homogenization by high pressure (French press), iv) homogenization by grinding (bead mill), and v) permeabilization by d…
Purpose
Protein purification is either preparative or analytical. Preparative purifications aim to produce a relatively large quantity of purified proteins for subsequent use. Examples include the preparation of commercial products such as enzymes (e.g. lactase), nutritional proteins (e.g. soy protein isolate), and certain biopharmaceuticals (e.g. insulin). Several preparative purifications steps are of…
Purification strategies
Choice of a starting material is key to the design of a purification process. In a plant or animal, a particular protein usually isn't distributed homogeneously throughout the body; different organs or tissues have higher or lower concentrations of the protein. Use of only the tissues or organs with the highest concentration decreases the volumes needed to produce a given amount of p…
Concentration of the purified protein
At the end of a protein purification, the protein often has to be concentrated. Different methods exist.
If the solution doesn't contain any other soluble component than the protein in question the protein can be lyophilized (dried). This is commonly done after an HPLC run. This simply removes all volatile components, leaving the proteins b…
Evaluating purification yield
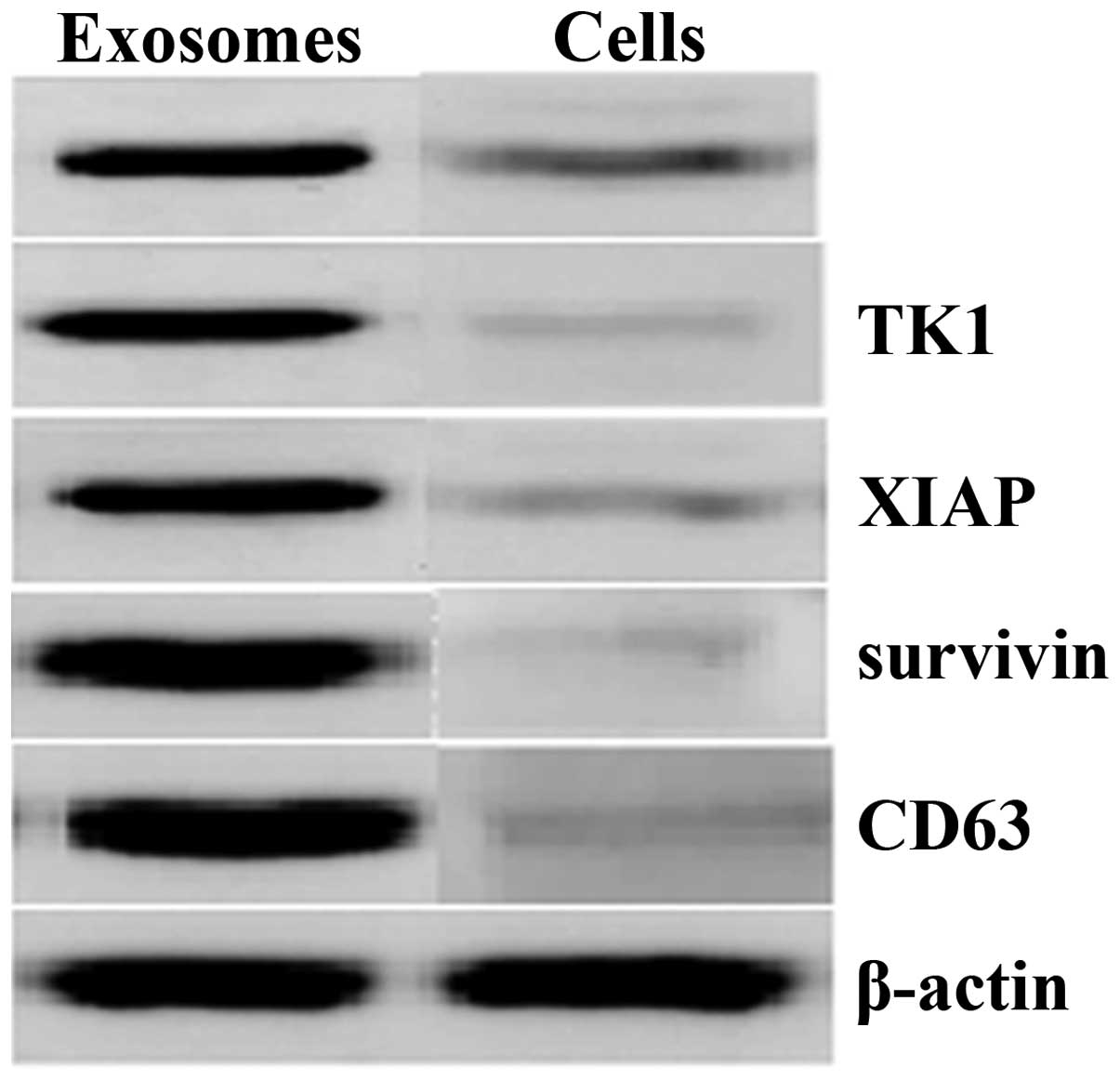
The most general method to monitor the purification process is by running a SDS-PAGE of the different steps. This method only gives a rough measure of the amounts of different proteins in the mixture, and it is not able to distinguish between proteins with similar apparent molecular weight.
If the protein has a distinguishing spectroscopic feature or an enzymatic activity, this property ca…
Analytical
Gel electrophoresis is a common laboratory technique that can be used both as preparative and analytical method. The principle of electrophoresis relies on the movement of a charged ion in an electric field. In practice, the proteins are denatured in a solution containing a detergent (SDS). In these conditions, the proteins are unfolded and coated with negatively charged detergent molecules…
See also
• Salting in
• Salting out
• Protein tag
• Protein production
• Host cell protein