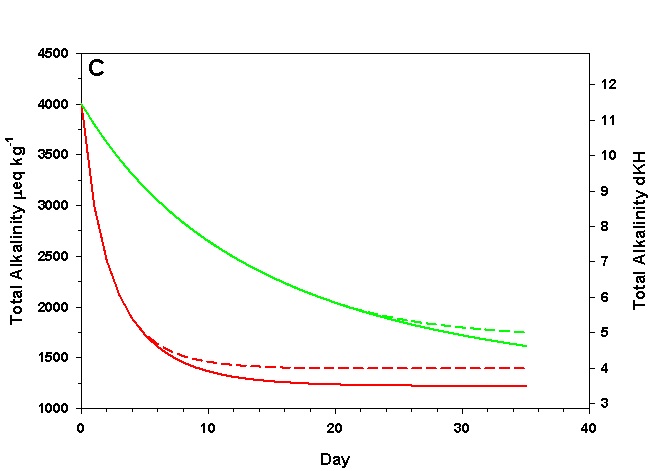
To dissolve calcium carbonate CaCO 3 (s) + H 2 CO 3 (aq) ⇌ Ca 2+ (aq) + 2HCO 3- (aq) To dissolve calcium carbonate: Increase pressure or decrease temperature Per the ideal gas law, this allows more CO 2 to enter the water The extra CO 2 creates more H 2 CO 3 There is too much H 2 CO 3 for equilibrium
What happens when CaCO3 reacts with rainwater?
However, the CaCO3 molecules react with rainwater resulting in the formation of soluble calcium bicarbonate or hydrogen carbonate of calcium. The reaction occurs due to the presence of carbon dioxide that gets dissolved in the rainwater from the atmosphere. This decreases the pH of water due to the formation of carbonic acid.
Is CaCO3 soluble in water?
Is CaCO3 Soluble in Water? Calcium carbonate is a chemical compound denoted by the chemical formula CaCO3. It is a carbonic salt of calcium and is also known as calcite or aragonite which are its mineral ores. It is commonly found in rocks and also forms the main component of seashells, eggshells, snail shells, pearls, corals, etc.
What happens when calcium carbonate reacts with water?
Under normal circumstances, calcium carbonate does not react with water. When CaCO3 is added to water it results in precipitation of most calcium carbonate molecules on the bottom with only a negligible amount of molecules getting dissolved.
What is the relationship between ω and CaCO3?
Ω tells us how CaCO3 will behave. but calcium carbonates are not constantly precipitating. In the ocean, Ω decreases with depth. Ω = [Ca 2 +] [ CO 3 2 −] [ CaCO 3] = 1 10 8.35 = 10 − 8.35

How do you reduce CaCO3?
The general recommendation to prevent CaCO3 precipitation is to lower the water pH by acid injection. Using chemical can be very expensive. Also, chemicals are harmful for environment and health. Magnetic process as a physical method can be alternative to the chemicals in order to decrease precipitation of CaCO3.
Can CaCO3 be dissolved in water?
So, is CaCO3 soluble in water? No, CaCO3 is insoluble in water because of the very strong electrostatic forces of attraction that exist in the calcium carbonate molecules.
What happens to CaCO3 in water?
CaCO 3(s) + 2 H +(aq) → Ca2+(aq) + CO 2(g) + H 2O(l) Calcium carbonate reacts with water that is saturated with carbon dioxide to form the soluble calcium bicarbonate.
What are factors that affect the solubility of calcium carbonate in water?
The order of importance of the other factors is temperature, salinity, and hydrostatic pressure. For water in equilibrium with the atmosphere, a condition probably only rarely attained, changes in temperature have the greatest effect on the solubility.
How do you increase the solubility of calcium carbonate in water?
Hence, in the dissociation equilibrium reaction of the calcium carbonate, calcium and carbonate ions are removed in the form of soluble salts. Hence, the molar solubility of calcium carbonate increases when sodium hydrogen sulphate is added.
How do you remove carbonate from water?
softening through carbonate removal. When the water has a high TH accompanied by a significant M-alk., the water can be softened by using lime to remove the carbonate.
What is caco3 in water?
Calcium carbonate appears as white, odorless powder or colorless crystals. Practically insoluble in water. Occurs extensive in rocks world-wide. Ground calcium carbonate (CAS: 1317-65-3) results directly from the mining of limestone.
What happens to calcium carbonate when heated?
If a compound is heated it will break down to two or more substances and these can be elements or compounds. So, if we have calcium carbonate and we heat it, it breaks down to produce calcium oxide and carbon dioxide.
Is CaCO3 soluble or insoluble?
insolubleCalcium carbonate appears as white, odorless powder or colorless crystals. Practically insoluble in water.
Is CaCO3 aqueous or solid?
Calcium carbonate (CaCO3) is insoluble, so it is a solid in water. When carbonate combines with metals in the second group of the periodic table, the compounds are generally insoluble.
Why is calcium bad for your pipes, and how does it get in your water?
You might be wondering how calcium can get into your water. You should know that there are numerous ways to enter your pipes and become a problem.
How to reduce water hardness?
In this method, we reduce water hardness by using cation resins. The cations used are usually resins that have strong-cation / strong-acid (SAC) resin beads. The process also helps to increase the concentration of sodium in the water. Scale-forming magnesium (Mg2+) and calcium (Ca2+) ions even are exterminated in the process.
What are the Best Reusable Filters for Removing Calcium From Water?
Water softeners mean that it is unlikely that you will need to buy the best reusable filters for removing calcium from the water.
Why is calcium present in water?
Calcium is naturally seen in the water. This may dissolve rocks like limestone, calcite, marble, gypsum, dolomite, apatite and fluorite.
What is limescale in water?
Defining it merely, Limescale is the chalky, white residue left behind from the activity of magnesium, calcium, and other dissolved minerals present in the water. Usually, it forms a hard white crust around the mouth of your bathroom or kitchen taps.
How much calcium is in 8 oz milk?
A glass of eight-ounce milk contains 300 milligrams of 1000 milligrams of calcium suggest for the people whose age is from 19 to 50. For the age 9 to 18, the requirement goes up to 1300 milligrams for a day as the body is growing very fast.
How to determine if water is hard?
You can measure water hardness by the volume of the mineral-deposits contained in the water. Soft water will have a calcium concentration of fewer than seventeen milligrams per liter. If the water contains between seventeen to sixty milligrams of calcium per liter , you can consider it to be slightly hard.
How to remove calcium from water?
Reverse osmosis is a process that removes calcium and other minerals from hard water. A faucet attachment is the easiest option if you simply want to remove calcium from your drinking water to improve the taste. Boil drinking water to soften it for drinking. Boil a pot of water for 10 minutes and then let it cool.
What is the best way to prevent calcium buildup in water?
Pick out a salt-based water softener system to install. Salt-based water softener systems are the best option to prevent calcium buildup and filter calcium out of your water supply. Talk to the professionals at your local retailer or research online to find a water softener that suits your budget and plumbing system.
How to soften water for drinking?
2. Boil drinking water to soften it for drinking. Boil a pot of water for 10 minutes and then let it cool. When you boil water you will remove some types of calcium mineral deposits, known as carbonate hardness, but not all types.
How long does a salt water softener last?
A salt-based water softener will last for many years and the only ongoing cost is to refill the salt in the tank.
What is the hardness of water that you can boil out of?
Carbonate hardness that you can boil out of drinking water includes calcium carbonate, calcium bicarbonate, and calcium hydroxide.
Where is the water shut off valve located?
The water supply shutoff valve is usually located on the inside of a wall near an outdoor faucet on the other side of the wall.
What is the best way to purify water?
Use a Brita, or other type of carbon water filter, to purify drinking water when all you want to do is improve the taste. This will not remove calcium, but will remove chlorine and other chemicals and improve the taste of your water. Remember that calcium is actually a mineral that is good for you!
How to lower CaCO3?
CaCO3 = Ca++ + CO3-- In this case one can lower the concentrations of either Ca++ or CO3--. The first can be done with a chelating agent such as EDTA or by adding an anion such as PO4--- that forms a more insoluble salt with Ca++. The second can be done by adding an acid such as HCl or acetic acid [vinegar] or even carbon dioxide to change CO3-- to HCO3- or by adding a cation such as Ba++ or Pb++ that form a carbonate less soluble than CaCO3. In any case after the reaction is complete the CaCO3 has been changed.
How to remove CaCO3 from water pipes?
So if some CaCO3 has formed a precipitation in lets say water pipes in your house or in a steam boiler, you can remove it by circulating an acid solution trough those pipes , but it is a “reaction” not “dissolving”, in chemical terms.
What is the reaction of calcium carbonate?
I’m not sure I would use the term “dissolve.” I’d go for “react.” Calcium carbonate undergoes a chemical reaction with hydrochloric acid. The reaction results in the formation of carbon dioxide gas and leaves calcium ions and chloride ions in solution. Just about any carbonate salt will react with just about any acid to liberate CO2 gas.
What is the solution that produces stalagmites and stalactites in limestone caves?
Slightly - it will form calcium hydrogen carbonate solution (temporarily hard water) - this is responsible for producing stalagmites and stalactites in limestone caves.
How much calcium carbonate dissolves in water?
However, in real life, if you measure very carefully, it turns out that a very tiny amount of the calcium carbonate DOES dissolve - about 15 milligrams per liter of water you soak the marble in. And if the water is at all acidic, like if there is acid rain, then the calcium carbonate will dissolve a lot. So over time, marble gravestones and statues and buildings do erode away from being out in the rain. It just takes a really long time.
What is the name of the salt that dissolves in water?
Carbonates are salts that become water soluble if an acid is added to it. Carbon dioxide is given off and, therefore, the carbonate-based rock dissolves. If, for instance, the rock is limestone (calcium carbonate, basically), it dissolution in water goes on like this: CaCO₃ + 2HCl → CaCl₂ + H₂O + CO₂.
What is the reaction that coats the outside of calcium carbonate?
Sulfuric acid : coats the outside of your calcium carbonate with insoluble calcium sulfate as the reaction proceeds, so it can’t go to completion without very finely powdered calcium carbonate or extensive mechanical agitation.
What is the first reaction with lime to produce hydrogen carbonate?
However, as in most cases water contains free CO 2, this will be the first to react with the lime to produce hydrogen carbonate:
What happens to precipitation in the absence of crystallisation seeds?
in the absence of crystallisation seeds, precipitation will be very slow, water will remain supersaturated and precipitation of the few suspended substances will tend to take place but mainly over available surfaces, especially if they are metal surfaces (reactor walls, agitators, channels, valves…).
Does caustic soda reduce water hardness?
Using caustic soda will, therefore, lower water hardness to a level that is equal to twice the reduction in bicarbonates belonging to the alkaline-earths.
Is magnesium carbonate soluble in water?
As magnesium carbonate is relatively soluble (approximately 70 mg ·L –1 ), an excess of lime will lead to the following reaction: And if amounts of reagent are precisely adjusted, the water’s alkalinity will be reduced to the theoretical solubility applicable to the CaCO 3 + Mg (OH) 2 system which ranges from 2 to 3°F under normal temperature ...
Does caustic soda have to be adjusted?
In practice, the amount of caustic soda injected into water must be adjusted so that we obtain a minimum amount of residual M-alk..
Does precipitation occur in crystals?
conversely, in the presence of crystals, precipitation takes place very fast and a balance is achieved within a few minutes. This applies at least when the surface of the available crystals remains sufficiently "clean": no impurities such as adsorbed organic polymers, colloids, metal hydroxides. In particular, when Mg (OH) 2 is precipitated, it will tend to slow precipitation down and above all to significantly lighten the crystal aggregates that have formed. Therefore, the reactor and separator used will have to be bigger, see chapter flocculators – settling tanks – flotation units.
Can you precipitate calcium and magnesium?
This concerns water with sodium alkalinity. We can always achieve satisfactory calcium and magnesium precipitation by calculating lime on M-alk. + C. However, this method will produce water that is loaded with caustic soda and it may be advisable to use smaller amounts. This comes down to only precipitating CaCO 3 in order to remove the ion that causes the greatest scaling:
Is calcium carbonate acidic?
Calcium carbonate will dissolve quickly in a strong acid, like hydrochloric, even if it is dilute and therefore not so dangerous.
Can organic solvent dissolve chalk?
Perhaps the problem is with the other materials, which may be binders (as Buttonwood suggested). Perhaps an organic solvent could dissolve the binder and thus remove the chalk.
Is C a C O X 3 soluble in water?
3. C a C O X 3 is sedimenting when mixed with water, and therefore is not easy to remove with hot water. Nevertheless several methods exist that actually dissolve it that are better than vinegar: You could add hydrochloric acid again to convert it into soluble Calcium chloride, according to equilibrium: C a C O X 3 ( s) + 2 H C l ( a q) ...
What is the sequence of carbonate removal?
The treatment has been designed on the basis of the following sequence: clarification – carbonate removal (lime) – filtration – polishing (O3 + GAC).
How to soften water with high TH?
softening through carbonate removal. When the water has a high TH accompanied by a significant M-alk., the water can be softened by using lime to remove the carbonate. This carbonate removal can be carried out: "catalytically" by Gyrazur when no concomitant clarification is needed and when the magnesium content is low; ...
How many m3 is Hanningfield DWTP?
Figure 47. Schematic diagram of the treatment applied at the Hanningfield DWTP (UK) – Capacity: 230,000 m3 · j–1
How hard is Hanningfield water?
The Hanningfield (UK) plant (230,000 m 3 ·d –1) processes reservoir water that, in addition to significant levels of micro algae, organic matter and pesticides, etc., is too hard (24 °F to 42 °F).
What is the advantage of softening?
The advantage of this type of softening is that it does not generate solid waste and that it can be carried out under pressure.
How to re-intain M-alk?
will have to be re-instated by mixing the water with a fraction of water from which the carbonates have not been removed.
Is water a modified anions?
The anions are not modified. Water obtained in this way has a zero TH: it is corrosive and unpleasant to the palate; a certain residual TH needs to be maintained (8 to 15°F) by only softening part of the output which is then mixed into the remaining output.
How to dissolve calcium carbonate?
To dissolve calcium carbonate: Increase pressure or decrease temperature. Per the ideal gas law, this allows more CO 2 to enter the water. The extra CO 2 creates more H 2 CO 3. There is too much H 2 CO 3 for equilibrium. The reaction must go to the right to get rid of the extra H 2 CO 3. CaCO 3 dissolves.
When does CO2 dissolve in water?
When pressure is higher, more CO2 dissolves into the water . When temperature is lower, more CO2 dissolves into the water. (At lower pressures and higher temperatures, CO 2 can escape as a gas.) Note that carbon speciates in the water depending on the water pH:
How can we decrease pressure?
So if you increase kinetic energy (waves), then pressure must go down.