Flame tests
| Ion present | Flame test colour |
| Lithium, Li+ | Red |
| Sodium, Na+ | Yellow |
| Potassium, K+ | Lilac |
| Calcium, Ca2+ | Orange-red |
How does a flame test identify a substance?
During a flame test, chemists take an unknown metal and put it under a flame. The flame will turn different colors based on which metal is in the substance. The scientists can then identify their unknown substance. Let's take a detailed look at how this works.
Which metal ion produces a different flame test colour?
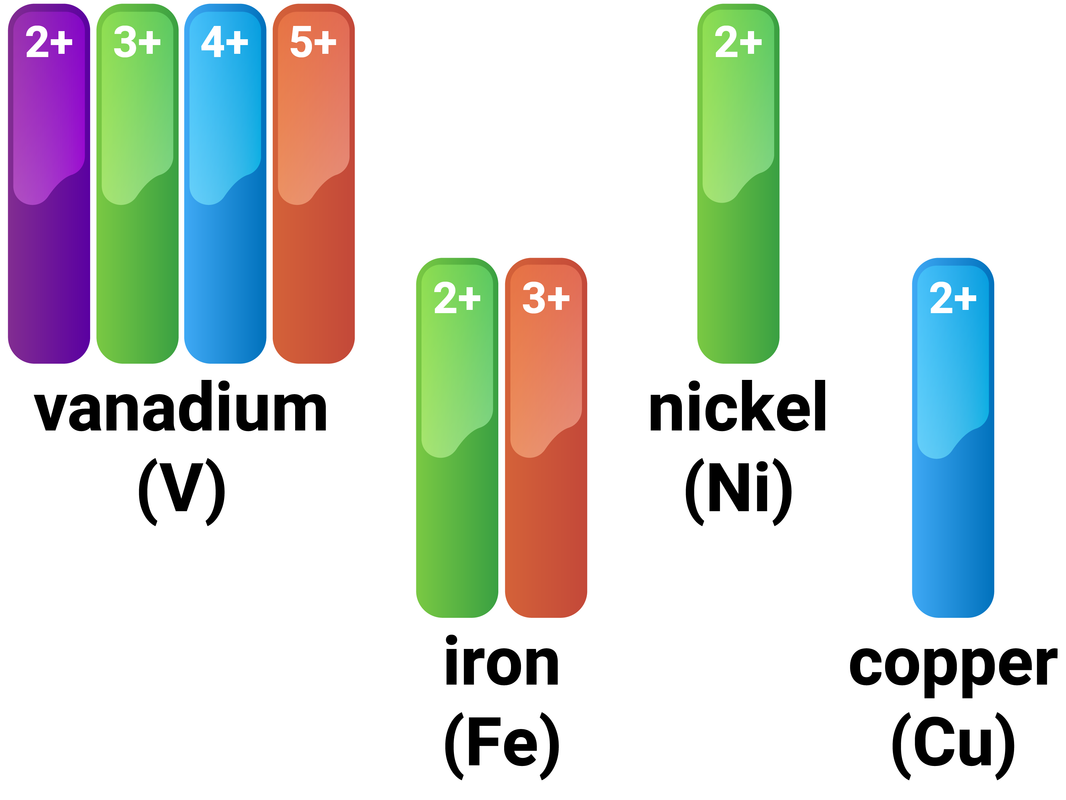
The metal ion is calcium, Ca2+. Each metal ion produces a different flame test colour.
How can you tell which ion is present in a flame?
In order to confidently identify which ion is present, the result for a test should be unique, and not caused by another ion. If a mixture of ions is present, some of the flame colours may not be clearly visible.
What happens to the electrons in a flame test?
As it turns out, this is exactly what happens during the flame test. During the flame test, metal ions are exposed to thermal energy, or heat, from the flame. The electrons in the outer orbital absorb the energy and temporarily bounce to a higher energy level. However, the electrons don't stay put there.

How can metal ions be identified using a flame test?
Different metal ions produce different flame colours when they are heated strongly. This is the basis of a flame test . In order to confidently identify which ion is present, the result for a test should be unique, and not caused by another ion.
Can flame test be used for metals?
A flame test is an analytical procedure used in chemistry to detect the presence of certain elements, primarily metal ions, based on each element's characteristic emission spectrum.
How can metal ions be identified?
Testing for cations is a test used in chemistry to identify metal or metal ions (cations) found in compounds. There are two types of tests used in chemistry to test for cations. The Flame test involves exposing the compound to a flame and identifying the compound by the flame color produced.
What can a flame test be used to identify?
The flame test is a qualitative test used in chemistry to help determine the identity or possible identity of a metal or metalloid ion found in an ionic compound. If the compound is placed in the flame of a gas burner, there may be a characteristic color given off that is visible to the naked eye.
Would flame tests be useful for detecting metal ions in a mixture?
Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Not all metal ions give flame colors. For Group 1 compounds, flame tests are usually by far the easiest way of identifying which metal you have got....Flame Tests.ElementcolorLeadgray-white9 more rows•Aug 15, 2020
Would the flame test be useful for detecting metal ions in a mixture of metal ions?
Flame tests are utilised in chemistry to identify the metal ions in compounds. They are more useful for some metals than others; particularly for the Group 1 metals, they provide a good way of quickly identifying the metal ion present.
How are flame tests used to identify cations?
0:552:31CTSC practical experiment: Identifying cations by means of a flame testYouTubeStart of suggested clipEnd of suggested clipAnd hold the wire with the salt in the flame. Observe the color of the flame. And determine whichMoreAnd hold the wire with the salt in the flame. Observe the color of the flame. And determine which cation is present clean the wire as before and repeat the test using the other salts which are listed.
How do you identify a metal ion in aqueous solution?
Dilute sodium hydroxide solution reacts with some metal ions in solution , forming metal hydroxides. Some of these metal hydroxides are insoluble , so they appear as precipitates . Copper hydroxide forms a blue precipitate.
How do you identify an ionic compound?
0:044:17How to identify ionic compounds and covalent compounds? - Dr KYouTubeStart of suggested clipEnd of suggested clipAnd how to identify. Them. Basically there are two types of compounds which are ionic and covalentMoreAnd how to identify. Them. Basically there are two types of compounds which are ionic and covalent compound ionic compound is formed from the attraction between cation.
What causes flame Colours of metal ions?
The color of a flame test is due to electrons in the metal cations becoming excited and jumping up to a higher energy level. This is unstable, so the electrons immediately return to their ground state. In doing so, they give off energy, some of which is in the visible light spectrum.
Flame Test
Take a lookup into the sky during a firework show and just imagine; each and every one of the vibrant colors that can be seen is the direct result of a specific chemical being ignited. There are several different metal ions inside of the firework that when ignited, release an array of different colors.
How Does The Flame Test Work?
Flame tests rely on the unique electron configurations of each and every element in order to identify them. When in compounds as these elements often are, they exist in the form known as an ion. A metal ion exists when the atom releases its valence electrons to bond with a non-metal atom as an ionic bond.
How to test for flame color?
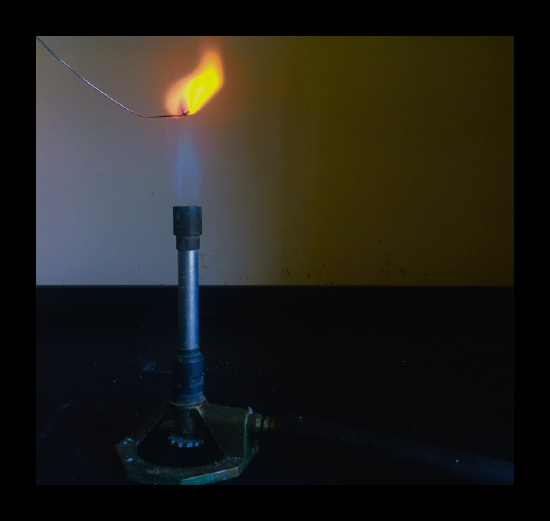
This is the basis of flame tests. To carry out a flame test: dip a clean wire loop into a solid sample of the compound being tested. put the loop into the edge of the blue flame from a Bunsen burner. observe and record the flame colour produced.
What is the purpose of flame test?
Tests for ions. Flame tests and chemical tests are used to detect and identify ions in samples. Instrumental methods of analysis are faster, and more accurate and more sensitive than simple chemical tests. Part of.
What is flame testing?
Analysing substances. Flame tests and chemical tests are used to detect and identify ions in samples. Instrumental methods of analysis are faster, and more accurate and sensitive than simple chemical tests. Part of. Chemistry (Single Science) Chemical analysis.
Why do metal ions produce different flame colors?
Different metal ions produce different flame colours when they are heated strongly. This is the basis of a flame test. In order to confidently identify which ion is present, the result for a test should be unique, and not caused by another ion. To carry out a flame test:
Why do we use flame test?
A flame test can be used to assist in identifying metal ions – where most will produce a strong colour when held under a consistent flame. The colour of the flame is subsequently used to identify the metal cations present in an unknown sample. The method to determine this can be seen below:
What is the test for anions and cations?
There are many available tests for anions and cations – most are precipitation reactions. Metal ions can combine with the hydroxide ions (OH–) from including a sodium hydroxide or ammonia solution. The insoluble precipitation with defining colours. The method to test is:
What is a flame test?
Flame Tests. Flame tests can be used to identify metal ions ( cations) in compounds. Steps to produce flame colours include: wearing eye protection, clean a wire loop by dipping in hydrochloric acid, and then holding in a blue flame for a few seconds.
How to test for sulfate ions?
Barium sulfate will form, which is an insoluble white precipitate. To test for sulfate ions in solution: add a few drops of dilute hydrochloric acid to the sample. add a few drops of dilute barium chloride solution.
What is a flame photometer reading?
A reading can be taken from the flame photometer for different concentrations of a metal ion in solution. These readings are then used to plot a calibration curve. Having a calibration curve of known samples, allows scientists to be able to identify the concentration of a sample just by looking at the graph!
How to test for halide ions in solution?
To test for halide ions in solution: add a few drops of dilute nitric acid to the sample. add a few drops of dilute silver nitrate solution. observe and record the colour of any precipitate that forms. We add acid first, because carbonate ions will also produce a white precipitate with silver nitrate solution.
How to detect CO 32?
Carbonate ions, CO 32- are detected using any dilute acid (e.g. hydrochloric acid). Bubbles of carbon dioxide gas will be given off when an acid is added. We can pass the gas through limewater and if it turns the limewater cloudy it will confirm the gas is carbon dioxide.
What happens when two chemicals react?
If we react two chemicals together and a new solid is made (one that doesn't dissolve in the solution) we call it a precipitation reaction . Compounds can dissociate (split apart into ions) in solutions, and the positive ions are referred to as cations.
What is the spectrum of an element?
the resulting pattern is known as a spectrum. Every element has its own specific set of wavelengths, so every pattern is unique (can be thought of as an element's 'fingerprint'). If there is a mixture of ions, one colour may be covered up by another in a flame test.