
PROCEDURE
- Mechanically increase resolution potential by decreasing particle size.
- Mechanically increase resolution potential by increasing column length.
- Chemically change the resolution potential by adjusting the mobile phase composition.
- Chemically change the resolution potential by changing to a different solvent in the mobile phase.
How can I improve peak resolution in column chromatography?
If the peak separation increase is less than the increase in peak width, peak resolution is degraded. This is usually the case for later eluting peaks (i.e., partition ratios [k] of around 10 or more) in the original chromatogram. Lowering column temperature is a common method to improve peak resolution, especially for early eluting peaks.
What is efficiency and resolution in column chromatography?
Efficiency and resolution. There are two features of the concentration profile important in determining the efficiency of a column and its subsequent ability to separate or resolve solute zones. Peak maximum, the first, refers to the location of the maximum concentration of a peak.
What is the resolution of a chromatogram?
The results from a chromatogram usually display peaks, and the resolution is how much two peaks are able to be distinguished from each other. Resolution is an important factor to consider when designing an experiment involving chromatography, and as an equation, resolution can be displayed as where t = retention times, and w = widths of the peak.
What are the basic chromatography parameters?
The resolution, capacity factor, retention factor, column efficiency, and selectivity are the basic chromatography parameters. Resolution is determined by three important parameters such as efficiency, selectivity, and retention. In this case, there is no specific parameter to apply, since each case is a special case in chromatography.
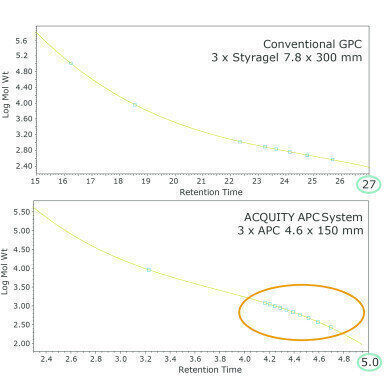
What can you try to improve the resolution in your gas chromatography method?
Increasing the carrier gas linear velocity will increase the speed of analysis. Loss of resolution can occur if the speed is increased much higher than the optimal velocity for the carrier gas. Using hydrogen and higher linear velocity will improve efficiency.
How can you improve the separation of chromatography?
Depending on the situation, separations can sometimes be improved by increasing the column plate number, by using smaller particles or by increasing column length. The disadvantages of these approaches are higher operating pressures and increased separation times for longer columns.
How can the efficiency of column chromatography be improved?
Decreasing particle size thus is a useful method for improving column efficiency and providing better separations. changing to particles that are half as big, while keeping the column length the same, will double the performance, but increase the required pressure by a factor of four.
What factors affect the resolution of the peaks in gas chromatography?
Factors Governing the Resolution of peaks in the Gas ChromatogramBoiling Point. Boiling point is the temperature at which a liquid transforms into vapour under existing pressure conditions. ... Column Temperature. ... Polarity. ... Carrier Gas Flow Rate. ... Column Length. ... Column Diameter. ... Film Thickness.
Which factors affect resolution?
P- 515721 7 • Resolution can also be expressed in the Resolution Equation as a combination of the factors (separation, efficiency, and retention) that affect this value.
What factors affect chromatography?
What factors affect chromatography? Retention factor values in thin layer chromatography are affected by the absorbent, the solvent, the chromatography plate itself, application technique and the temperature of the solvent and plate.
Why is resolution important in chromatography?
Since resolution is a function of peak width and retention time, both of which are inherent properties of the chromatographic process, resolution measurements can be used for optimizing separations.
What is meant by resolution in chromatography?
In general, resolution is the ability to separate two signals. In terms of chromatography, this is the ability to separate two peaks. Resolution, R, is given by. where tr1 and tr2 and w1 and w2 are the times and widths, respectively, of the two immediately adjacent peaks.
What could have caused the column chromatography to have low resolution?
A fast flow rate leads to short retention times but can give poor resolution. A slow flow rate gives long retention times and broad peaks.
How does temperature affect resolution in gas chromatography?
The temperature is a very important parameter to influence separation. As a rule of thumb, for every 15 °C higher or lower, the retention of a column decreases or increases by a factor of 2. That means if the last peak elutes at 100 °C after 10 minutes, it will elute at 5 minutes at 115 °C and at 20 minutes at 85 °C.
What factors affect resolution in ion chromatography?
Size, size distribution and porosity of the matrix particles are the main factors which affect the flow characteristics and chromatographic resolution.
How can you improve the resolution between two closely spaced peaks in GC?
Lowering column temperature is a common method to improve peak resolution, especially for early eluting peaks.
What is column efficiency in chromatography?
Column efficiency, indicated as the number of theoretical plates per column, is calculated as N = 5.54 (tR / w0.5)2 where tR is the retention time of the analyte of interest and w0.5 the width of the peak at half height.
What will be effect of increase in column temperature on column efficiency?
If the column temperature is increased, the chromatographic separation process becomes faster and, in general, more efficient. However, the percentage decrease in retention time is usually not the same for all compounds of a sample mixture and changes in peak spacing are common.
What affects column chromatography?
Certain limiting factors affecting the column chromatography are nature of solvent, column temperature and pressure. The dimensions of the column and particle size of the adsorbent are also factors to consider [26].
Which of the following parameters can decrease column efficiency in liquid chromatography?
If the flow rate is too high, the mass transfer term (Cv) will increase and reduce column efficiency. At high flow rates the adsorption of the analyte to the stationary phase results in some of the sample lagging behind, which also leads to band broadening.
What is the resolution of chromatography?
In general, resolution is the ability to separate two signals. In terms of chromatography, this is the ability to separate two peaks. Resolution, R, is given by where tr1 and tr2 and w1 and w2 are the times and widths, respectively, of the two immediately adjacent peaks. If the peaks are sufficiently close, which is the pertinent problem, w is nearly the same for both peaks and resolution may be expressed as
What is the second feature of chromatography?
The second feature important to efficiency and resolution is the width of the peak. Peaks in which the maxima are widely disengaged still may be so broad that the solutes are incompletely resolved. For this reason, peak width is of major concern in chromatography.
What is the concentration profile of a column?
There are two features of the concentration profile important in determining the efficiency of a column and its subsequent ability to separate or resolve solute zones. Peak maximum, the first, refers to the location of the maximum concentration of a peak. To achieve satisfactory resolution, the maxima of two adjacent peaks must be disengaged. Such disengagement depends on the identity of the solute and the selectivity of the stationary and mobile phases.
What are the forces that attract solutes to the two phases?
The forces attracting solutes to the two phases are the normal forces existing between molecules — intermolecular forces. There are five major classes of these forces: (1) the universal, but weak, interaction between all electrons in neighbouring atoms and molecules, called dispersion forces, (2) the induction effect, by which polar molecules (those having an asymmetrical distribution of electrons) bring about a charge asymmetry in other molecules, (3) an orientation effect, caused by the mutual attraction of polar molecules resulting from alignment of dipoles (positive charges separated from negative charges), (4) hydrogen bonding between dipolar molecules bearing electron-pair-accepting hydrogen atoms, and (5) acid-base interactions in the Lewis acid-base sense—i.e., the affinity of electron-accepting species (Lewis acids) to electron donors ( Lewis bases ). The interplay of these forces and temperature are reflected in the partition coefficient and determine the order on polarity and eluotropic strength scales. In the special case of ions, a strong electrostatic force exists in addition to the other forces; this electrostatic force attracts each ion to ions of opposite charge. This is an important element of ion-exchange chromatography.
What determines the rate of migration of substances in chromatographic procedures?
The rates of migration of substances in chromatographic procedures depend on the relative affinity of the substances for the stationary and the mobile phases. Those solutes attracted more strongly to the stationary phase are held back relative to those solutes attracted more strongly to the mobile phase.
Is the force of attraction stronger for one solute than another?
The forces of attraction are usually selective—that is to say, stronger for one solute than another. At least one of the two phases must exert a selective effect, and very often both phases are selective, as in liquid and supercritical-fluid chromatography.
Why does peak resolution change?
Changes in resolution are due to changes in peak separation and/or peak width. Decreasing column temperatures usually increase peak separation but often with a corresponding increase in peak width. If the increase in peak separation is greater than the increase in peak width, improved peak resolution occurs.
What is the maximum resolution of hydrogen?
With hydrogen as carrier gas the linear flow velocity for maximum resolution is 50 cm/s. Increasing the flow will decrease resolution slightly, but will save time. Decreasing the flow will degrade resolution and waste time, so is pointless.
What are the parameters to adjust the resolution of a FID detector?
You have two parameters to adjust in order to improve the resolution. One of them you have already "touch". It is the temperature ramp. this parameter may vary the resolution but in very small amount. The parameter who adjust the resolution more drastically is the column flow. It depends not only of the flow value but also depends on the carrier gas. The best carrier gas is hydrogen, and the best compromise is Helium. Also, if you are using a FID detector, one important parameter to be taken into account is the makeup gas flow. This parameter is a flow value from the same carrier you are using to analyze your samples declared for the detector.
What to do if you have the budget and the possibility to change the configuration of your GC?
If you have the budget and the possibility to change the configuration of your GC, try heartcutting or GCxG C.
What is the df of a 30-40 m column?
suggest the use of a shorter column of the one you are using but with a film thickness of the stationary phase slightly larger (50-60 me df = 0.5 -1 micrometers). You can use an even shorter column of 30-40 m with an inner diameter of 100 micrometers and df = 0.2-0.5 micrometers. Uses the H2 as carrier and a programmed temperature. The results should be positive. Otherwise you may use the columns in series. This work you find on the RS.
Why is it possible to achieve when one or more derivatizing agents are used?
Another solution, not so practical, it is possible to achieve when one or more derivatizing agents in order to enhance volatility of the compounds and thus promote the formation of narrower chromatographic peaks.
Does decreasing the flow rate help?
Decreasing the flow rate might help or if you are using nitrogen as a carrier gas than use helium. Also if your peak respose is good than increasing the split ratio will decrease the peak response and thus the co-eluting peak might get seperated.
What is resolution in chromatography?
The resolution is determining the ability to separate two analytes. It is a measure of how well two elution peaks can be differentiated in chromatographic separation.
What are the three parameters that determine resolution?
Resolution is determined by three important parameters such as efficiency, selectivity, and retention . In this case, there is no specific parameter to apply, since each case is a special case in chromatography.
Can you change the conditions to get better separation?
If you know that there is a difference in the structure between the molecules you want to separate, you can change the conditions accordingly to get better separation. If you do not have the compound identity, then you have to rely on your experience to make the appropriate changes for a better solution.
Does gas chromatography change with temperature?
Because in gas chromatography (GC) you can change with the column, flow rate and temperature gradient, the concentration of the mobile phase is always the same. As you know, liquid chromatography consists of separations according to the chemical interactions between the analytes and the stationary phase (column).
