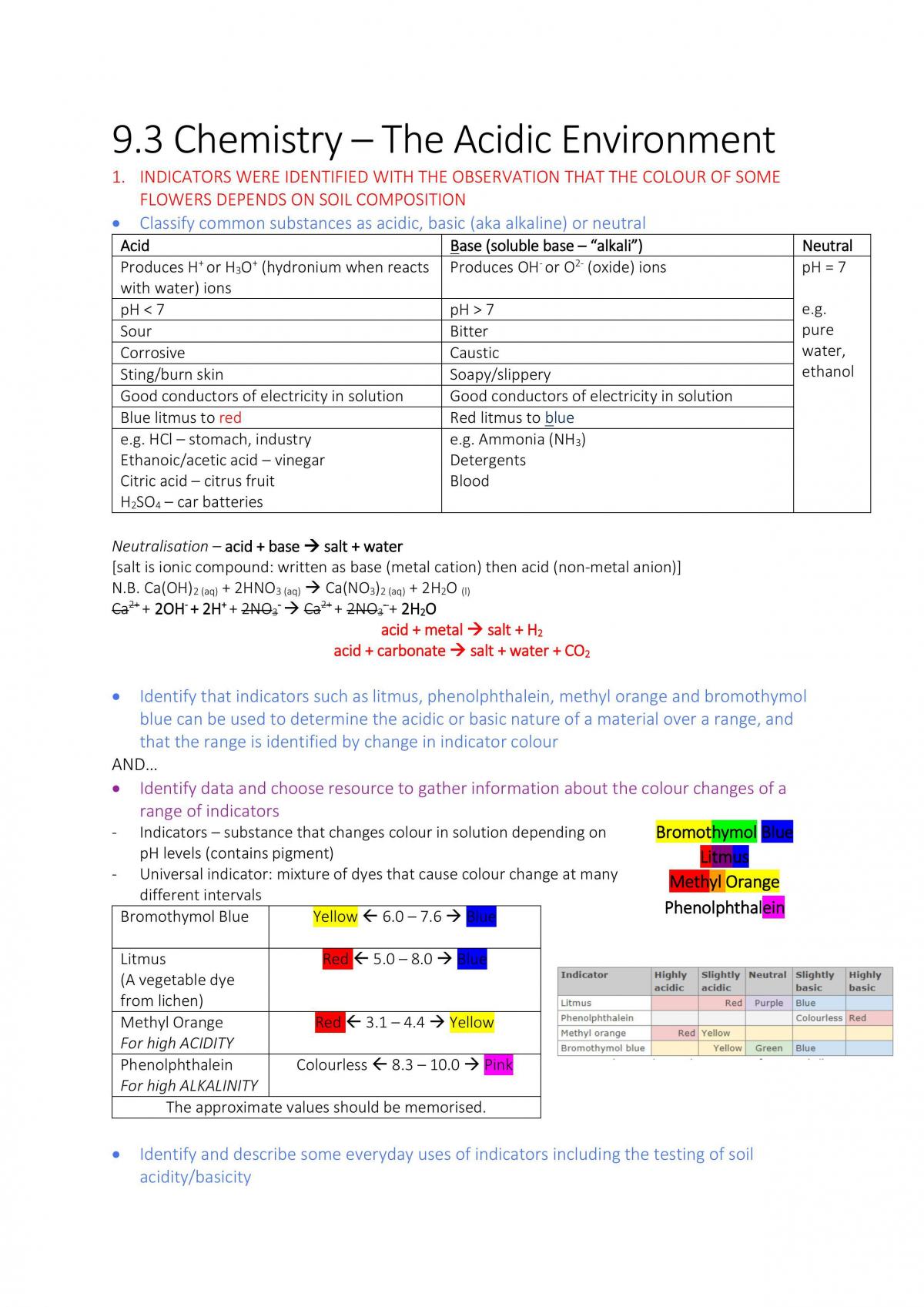
How do acids and bases affect the environment?
How Do Acids and Bases Affect the Environment? Acids and bases affect the environment by altering habitats so that they are more favorable to some organisms than others. Acids and bases naturally occur in the environment, and organisms have adapted to the pH of their local habitat over evolutionary time.
How does acid rain affect plants and animals?
Acid rain affects aquatic environments such as streams, lakes, and rivers. Acid rain builds up in these bodies of water, resulting in acidification. This can negatively affect marine plants and animals. It can also remove nutrients from soil, weakening trees and plants from the roots, and ultimately killing them.
How does acidity affect aquatic ecosystems?
This alone damages aquatic organisms; however, an increase in acidity also causes aluminum that is found in soil to be leached into bodies of water. The combined impact of aluminum and acidity causes damage to aquatic ecosystems. Example: many fish eggs cannot hatch at a pH of less than 5.
What are the causes of acid rain?
While erupting volcanoes can cause acid rain, the main culprits are nitrogen oxides, sulfur dioxide, and carbon dioxide, which are released into the atmosphere from vehicles, power plants, and manufacturing plants. Let's check out the reactions that create acid rain.
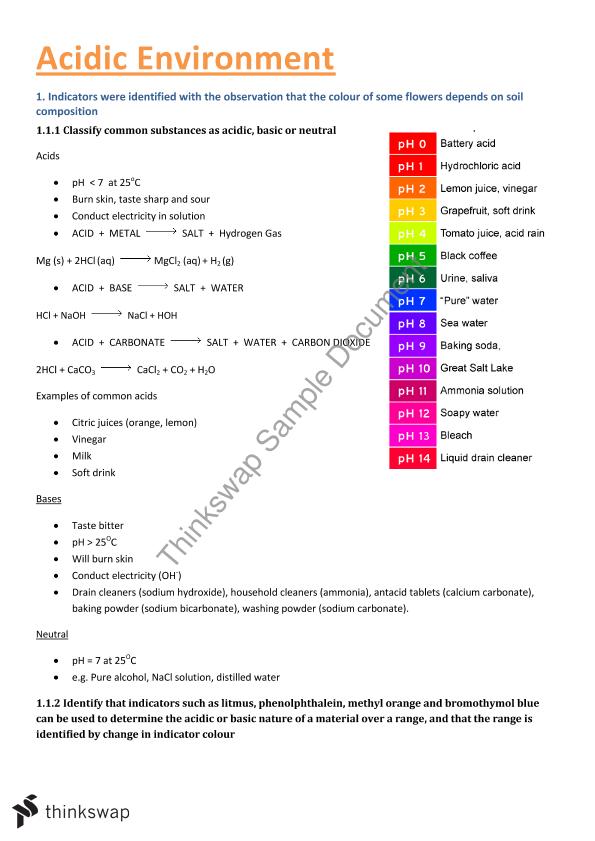
How does acids affect humans and the environment?
Not only are plants affected by acid deposition, but humans are too. If we breathe in the infinitesimal acid particles, we are prone to getting lung and respiratory problems and diseases such as asthma, chronic bronchitis (long-term), and pneumonia.
Can acid cause pollution?
Before falling to the earth, acid-causing emissions (sulphur dioxide and nitrogen oxide gases and the related acid particles) contribute to haze and smog and affect public health.
What are 3 effects of acid rain on the environment?
It has been shown that acid rain has detrimental effects on trees, freshwaters and soils, destroys insects and aquatic life-forms, causes paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and sculptures, as well as impacts on human health.
What are the problems with acid?
Heartburn, regurgitation, and dyspepsia are a few of the most common acid reflux symptoms. Heartburn. Also called acid indigestion, heartburn is a burning pain or discomfort that can move up from your stomach to the middle of your abdomen and chest. The pain can also move into your throat.
Why acid rain is harmful?
Acid rain can be extremely harmful to forests. Acid rain that seeps into the ground can dissolve nutrients, such as magnesium and calcium, that trees need to be healthy. Acid rain also causes aluminum to be released into the soil, which makes it difficult for trees to take up water.
How does acid rain cause pollution?
Acid rain is caused by a chemical reaction that begins when compounds like sulfur dioxide and nitrogen oxides are released into the air. These substances can rise very high into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants, known as acid rain.
How does acid rain affect water and soil?
Acid rain is thought to be acidifying soils and freshwaters, dissolving and releasing aluminum in concentrations toxic to fish and plants. Acid rain is also thought to be leaching nutrients from soils, thereby lowering their fertility.
Is acid rain harmful to humans?
While acid rain cannot harm humans directly, the sulfur dioxide that creates it can cause health problems. Specifically, sulfur dioxide particles in the air can encourage chronic lung problems, like asthma and bronchitis.
How does acid rain affect the atmosphere?
The effects of acid rain, combined with other environmental stressors, leave trees and plants less healthy, more vulnerable to cold temperatures, insects, and disease. The pollutants may also inhibit trees' ability to reproduce. Some soils are better able to neutralize acids than others.
Why can't I burp when I feel like burping?
Inability to burp or belch occurs when the upper esophageal sphincter (cricopharyngeus muscle) cannot relax in order to release the “bubble” of air. The sphincter is a muscular valve that encircles the upper end of the esophagus just below the lower end of the throat passage.
How does acid rain affect animals?
Acid rain can cause serious problems for many different animals and plants. As a result, the entire food web is affected. For example, acid rain can cause phytoplankton in lakes to die. Insects, which rely on phytoplankton for food, now have less food to eat, and they begin to die as a result.
What are the advantages and disadvantages of acid rain?
Acid rain affects the fresh water ponds and lakes and destroys the aquatic life as some species of fishes are rare and may be extinct. it can affect the trees particularly those that are high altitude. it can damage historical monuments and buildings.
What are acid pollutants?
acidic pollutants - harmful particles, such as sulfur dioxide and nitrogen oxides, that turn into acid when they mix with water and oxygen in the sky.
Is acid rain harmful to humans?
Walking in acid rain, or even swimming in a lake affected by acid rain, is no more dangerous to humans than walking in normal rain or swimming in non-acidic lakes. However, when the pollutants that cause acid rain —SO2 and NOX, as well as sulfate and nitrate particles— are in the air, they can be harmful to humans.
Is acid deposition a primary pollutant?
The types of pollutants that cause acid deposition are primary pollutants because once in the atmosphere, they react to form harmful substances.
Does air pollution result in acid deposition?
Acid deposition represents the mix of air pollutants that deposit from the atmosphere leading to acidification of soils and freshwaters. It mainly consists of pollutants emitted by the combustion of fossil fuels (e.g. power generation).
How do acids and bases affect the environment?
Acids and bases affect the environment by altering habitats so that they are more favorable to some organisms than others. Acids and bases naturally occur in the environment, and organisms have adapted to the pH of their local habitat over evolutionary time.
What happens if the pH of water changes?
For fish or frogs that live in small, isolated waters, a change in pH could kill the entire population. Acids and bases can also exacerbate the rate of erosion.
Why is soil pH important to terrestrial organisms?
The primary reason that soil pH is important to terrestrial animals is because soil pH influences which plants live in the area. Some plants thrive in high pH soils, while others thrive in low pH, or acidic, soils.
We believe in Compassionate Humanity
The majority of the emissions of sulphur dioxide and nitrogen oxides come from human activities such as burning of fossil fuels or vehicle exhaust fumes. However, a small fraction of emissions exist from natural processes such as decaying vegetation and volcanic activity.
About the Author
Sammy Witchalls has a bachelor’s degree in Environmental Science after studying at Lancaster University, UK and Monash University, Australia. Having previously worked for the Carbon Disclosure Project he is now working as an Urban Ranger where he restores river valleys in Manchester, UK.
What is acid rain, and how does it affect the environment?
Even though it basically sounds like a buzzword at this point, acid rain is a very serious matter. It refers to rain, snow, fog, dust, or hail that contains acidic components, such as sulfur or nitric acid.
Does acid rain still affect the planet?
Although acid rain persists in 2022, it isn't as big of an issue. So why is that the case?
What are the sources of SO2?
In the United States, electric utilities are the leading source of SO 2 emissions, while transportation sources generate the highest contribution of NO X. While the 1970 Clean Air Act empowered the U.S. Environmental Protection Agency (EPA) to regulate SO 2 and NO X emissions, federal action aimed specifically at reducing acid rain was not taken until 1990. The 1990 enactment of Title IV of the Clean Air Act established the acid rain program, including a market-based pollution reduction regime to address SO 2 emissions and the National Acid Precipitation and Assessment Program, which reports to Congress. Since its implementation in 1995, Title IV has led to a 40% reduction of SO2 emissions nationwide from electric utilities at only 25% of the projected cost. The picture for NO X emissions has been less encouraging, with only some sources and source categories showing reductions in emissions, while others have shown increasing contributions.
What is acid deposition primer?
An Acid Deposition Primer [PDF], prepared by NYSERDA , provides a more detailed overview of the science behind acid deposition and its effects on human health and the environment, as well as a summary of research findings in New York and the Northeast.
How do acids and bases affect the environment?
Acids and bases affect our environment significantly by altering it. These alterations may be beneficial in some cases, while in others, maybe really harmful. Acids and bases occur naturally in our environment, most commonly in our soil and water. Their presence changes the pH of the environment.
What causes pH to drop below 7?
Acids causes the pH to fall below 7, while bases increase it beyond 7, the change depending upon the amount of acids/bases, etc. Depending on the presence of acids and bases, soils may be acidic or basic or neutral and will thus support organisms (most commonly, the soil bacteria) that prefer those environments.
Does water have acidic or basic pH?
Similarly, water in natural streams (rivers, etc.) can also have acidic or basic or neutral pH and will, correspondingly, support specific life forms. Acids and bases can also be released into the environment by human activities. Acid rain is a direct effect of human activities and causes dissolution of rocks and minerals.
Acid rain forms when pollutants mix with moisture in the air and falls back as precipitation, adversely affecting the environment. If you want to know more specifically about how acid rain affects the environment, read on below!
Acid rain is a growing problem adversely affecting many parts of the world. But exactly how does acid rain affect the environment?
Acid Rain Effects on the Ecosystem
The most detrimental effect of acid rain is probably on the ecosystem. In ecology, an ecosystem is a community of different species of living organisms and their physical environment. The term can refer to different areas in the natural world, such as forests, deserts, or oceans.
Acid Rain Effects on Fish
Though the ecological effects of acid rain on fish and other aquatic life have been well documented, the full extent of the damage is still not fully understood, but it carries a variety of extremely harmful effects on fish.
Effects of Acid Rain on Plants
Acid rain is a type of pollution that can have harmful effects on plants and trees. Though it’s a form of pollution, it is itself caused by air pollution, such as when sulfur dioxide and nitrogen oxide get released into the atmosphere.
Acid Rain Effects on Humans and Animals
Acid rain is a serious environmental problem that can have a devastating effect on human and animal health. Acid rain can also fall on human and animal skin, causing irritation and potentially leading to skin cancer.
Effects of Acid Rain on Buildings and Structures
Acid rain can damage buildings and structures. The most common way that acid rain damages buildings are by the dissolution of calcium carbonate. Calcium carbonate is a major component of limestone, marble, and other types of stone.
How to Control and Minimize Acid Rain Occurrences
Now that you have learned how acid rain affects the environment, it would help to know how to minimize its occurrences.
How does acid rain affect the environment?from enotes.com
Acid rain is a direct effect of human activities and causes dissolution of rocks and minerals.
How does acid affect pH?from enotes.com
Their presence changes the pH of the environment. Acids causes the pH to fall below 7, while bases increase it beyond 7, the change depending upon the amount of acids/bases, etc. Depending on the presence of acids and bases, soils may be acidic or basic or neutral and will thus support organisms (most commonly, ...
What does it mean when an acid is weak?from sciencelearn.org.nz
This means that, for a strong acid like hydrochloric acid (HCl), the molecules ‘split’ to form H + ions and Cl – ions. Weak acids, such as ethanoic acid ( CH 3 COOH), do not fully dissociate. In fact, only about 1% ethanoic acid molecules split up to form H+ ions and CH 3 COO – ions at any one time.
What does "strong" mean in acid?from sciencelearn.org.nz
When talking about acids and bases, the words ‘strong’ and ‘weak’ have a very specific meaning and it’s not necessarily what you would expect. Strong acids dissociate completely in water to produce the maximum number of H + ions. This means that, for a strong acid like hydrochloric acid (HCl), the molecules. 18.
Why do acids taste sour?from sciencelearn.org.nz
Because acids can damage cells, our stomach#N#4#N#needs a special lining to protect it from the hydrochloric acid used to digest our food. We are familiar with some acids – citrus fruits, tomatoes and vinegar are acidic.
What happens when you put acid in water?from sciencelearn.org.nz
When we put a molecule of acid into water, it breaks apart. The science term for this is that it dissociates. For example hydrochloric acid (HCl) dissociates into hydrogen ions (H +) and chloride anions (Cl - ).
What are the reactions of acids?from sciencelearn.org.nz
Acids react with most metals including magnesium to create hydrogen gas and a salt – there are lots of different types of salts in chemistry. They also react with a group of substances called carbonates to produce carbon dioxide gas, salt and water. Learn about the reactions of calcium carbonate (like limestone) in this article.