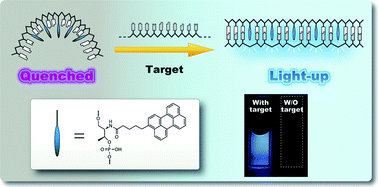
Fluorophores are fluorescent markers used to detect the expression of cellular molecules such as proteins or nucleic acids. They functionally accept light energy (for example, from a laser) at a given wavelength and re-emit it at a longer wavelength. These two processes are called excitation and emission.
What is fluorophore?
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation.
How does a fluorophore absorb light?
1) The fluorophore absorbs light energy of a specific wavelength. 2) Light absorption results in excitation of the fluorophore's electrons. 3) The fluorophore re-emits the absorbed light energy at a longer wavelength upon the electrons return to their basic state.
What is the excitation and emission of fluorophores?
Top: Excitation and emission fundamentals of fluorophores. 1) The fluorophore absorbs light energy of a specific wavelength. 2) Light absorption results in excitation of the fluorophore's electrons. 3) The fluorophore re-emits the absorbed light energy at a longer wavelength upon the electrons return to their basic state.
What can I do with a fluorophore?
Fluorophores can also be used to quench the fluorescence of other fluorescent dyes (see article Quenching (fluorescence)) or to relay their fluorescence at even longer wavelength (see article FRET ) See more on fluorescence principle .

What does a fluorophore do?
Fluorophores (or fluorochromes) are commonly used in conjugation with antibodies as detection reagents in applications such as flow cytometry. Fluorophores can absorb and emit light within a range of wavelengths, normally referred to as the absorbance (excitation) and emission spectra.
How are fluorophores attached to antibodies?
Fluorochromes can be covalently conjugated to antibodies through reactions with thiol or amine groups. Typically, fluorochromes containing isothiocyanate, succinimidyl ester, or sulfonyl chloride reactive groups are conjugated to amines on the antibody molecules.
How are fluorophores detected?
Detection strategies An excitation light source, such as lasers, photodiodes, or lamps (xenon arc and mercury vapor being the most commonly used lamps) A fluorophore. Filters to isolate specific wavelengths to excite different fluorophores. A detector that records the output, which is usually an electronic signal.
How does fluorescence work?
Fluorescence occurs when an excited molecule, atom, or nanostructure, relaxes to a lower energy state (usually the ground state) through emission of a photon without a change in electron spin. When the initial and final states have different multiplicity (spin), the phenomenon is termed phosphorescence.
How is a fluorophore excited?
A fluorophore is excited most efficiently by light of a particular wavelength. This wavelength is the excitation maximum for the fluorophore. Light with a wavelength near the excitation maximum can also cause excitation, as shown by the shaded areas below, but it does so less efficiently. Excitation range and maximum.
What's the difference between fluorescence and immunofluorescence?
Immunofluorescence indicates that a fluorescent tag was used to visualize the marker of interest but fluorescent markers can be used for immunocytochemistry (cells) or for immunohistochemsitry (tissues).
Why does fluorescence occur?
Fluorescence occurs when an atom or molecules relaxes through vibrational relaxation to its ground state after being electrically excited. The specific frequencies of excitation and emission are dependent on the molecule or atom.
What are fluorophores made of?
Biological fluorophores are commonly comprised of fluorescent proteins such as GFP. They have been used for cell labeling and characterization with varied rates of success. Samples stained with GFP emit bright green fluorescent signals when excited with ultraviolet incident light.
How is fluorescence imaging done?
In a fluorescent microscope, a sample is labeled with a fluorophore, and then a bright light (excitation light) is used to illuminate the sample, which gives off fluorescence (emission light). In this manner, samples are highly contrasted to the black background as the fluorophore emits a bright-colored light.
Why do fluorophores fluoresce?
Fluorophores are molecules that, upon absorbing light energy, can reach an excited state, then emit light energy. The three-stage process of excitation, excited lifetime, and emission is called fluorescence. Fluorophores absorb a range of wavelengths of light energy, and also emit a range of wavelengths.
Where does energy go in fluorescence?
The mechanism of fluorescence When the electrons relax to their ground state or another state with a lower energy level, energy is released as a photon. As some of the energy is lost during this process, light with an increased wavelength and lower energy is emitted by the fluorochrome compared to the absorbed light.
What are the three stages of fluorescence?
In short, the 3 steps of fluorescence are absorption (or excitation), non-radiative transition (or excited-state lifetime), and fluorescence emission.
Is fluorophore an antibody stain?
Here, fluorophore-conjugated secondary antibodies are used to bind target-specific primary antibodies that recognize the analyte of interest. The main advantage of using labeled polyclonal secondary antibodies is that they provide signal amplification since multiple secondary antibodies can bind each primary antibody.
Where is the fluorophore in GFP?
The principle fluorophore (often termed a chromophore) is a tripeptide consisting of the residues serine, tyrosine, and glycine at positions 65-67 in the sequence.
How does fluorescence microscopy work?
A fluorescence microscope, on the other hand, uses a much higher intensity light source which excites a fluorescent species in a sample of interest. This fluorescent species in turn emits a lower energy light of a longer wavelength that produces the magnified image instead of the original light source.
How are fluorescent antibodies made?
The fluorescent antibody technique consists of labeling antibody with dyes such as fluorescein isothiocyanate (FITC). These compounds have high affinity for proteins with which they conjugate.
Why do fluorophores fluoresce?
These fluorophores fluoresce due to delocalized electrons which can jump a band and stabilize the energy absorbed. Benzene, one of the simplest aromatic hydrocarbons, for example, is excited at 254 nm and emits at 300 nm. This discriminates fluorophores from quantum dots, which are fluorescent semiconductor nanoparticles .
What is a fluorophore?
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, ...
Why do fluorescent proteins have dark fractions?
Fluorescent proteins can have a dark fraction from protein misfolding or defective chromophore formation. These characteristics drive other properties, including the photobleaching or photoresistance (loss of fluorescence upon continuous light excitation).
What is a fluorophores marker?
More generally they are covalently bonded to a macromolecule, serving as a marker (or dye, or tag, or reporter) for affine or bioactive reagents ( antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, i.e., fluorescent imaging and spectroscopy .
How does a fluorophore absorb light?
The fluorophore absorbs light energy of a specific wavelength and re-emits light at a longer wavelength. The absorbed wavelengths, energy transfer efficiency, and time before emission depend on both the fluorophore structure and its chemical environment, as the molecule in its excited state interacts with surrounding molecules. Wavelengths of maximum absorption (≈ excitation) and emission (for example, Absorption/Emission = 485 nm/517 nm) are the typical terms used to refer to a given fluorophore, but the whole spectrum may be important to consider. The excitation wavelength spectrum may be a very narrow or broader band, or it may be all beyond a cutoff level. The emission spectrum is usually sharper than the excitation spectrum, and it is of a longer wavelength and correspondingly lower energy. Excitation energies range from ultraviolet through the visible spectrum, and emission energies may continue from visible light into the near infrared region.
What are fluorescent proteins?
Fluorescent proteins GFP (green), YFP (yellow) and RFP (red) can be attached to other specific proteins to form a fusion protein, synthesized in cells after transfection of a suitable plasmid carrier. Non-protein organic fluorophores belong to following major chemical families:
How many atoms are in a fluorophores?
Most fluorophores are organic small molecules of 20 - 100 atoms (200 - 1000 Dalton - the molecular weight may be higher depending on grafted modifications, and conjugated molecules), but there are also much larger natural fluorophores that are proteins: green fluorescent protein (GFP) is 27 k Da and several phycobiliproteins (PE, APC...) are ≈240k Da .
What is the vibrational level of a fluorophore?
Fluorophore. A fluorophore excited to some high-lying vibrational level of S1 or to higher singlet excited states rapidly relaxes (10−12s or less), in condensed phase, to the lowest vibrational level of S1 (Kasha's rule), by internal conversion and vibrational relaxation with energy transfer to the solvent.
How efficient are fluorochromes?
The most efficient fluorochromes have quantum efficiencies of ca. 30%, but their efficiency can be reduced by means of quenching processes such as photobleaching (see below). The time it takes for a molecule to fluoresce is of the order of the nanosecond. Switchable fluorophores that blink on and off have been developed.
How are NCs synthesized?
Generally, metal NCs are synthesized by reduction of metal ions in the presence of suitable reducing agents, similar to the synthesis of metal NPs. However, under such conditions, metal NCs are prone to strongly interact with each other, causing irreversible aggregation that reduces their surface energy, thus resulting in the formation of large NPs. Therefore, a suitable stabilizing scaffold is a necessity for producing metal NCs. The nature of the scaffold is responsible not only for their sizes but also for their fluorescence properties. Different kinds of scaffolds that can be used for the synthesis of metal NCs include DNA oligonucleotides, peptides, proteins, dendrimers, and polymers.
What is the purpose of GFP?
Another fluorophore that has become useful in the pH field is GFP, which is a natural product of the jellyfish Aequorea victoria, and typically used to label proteins expressed in cells ( 108, 353 ). Several researchers have engineered GFP mutants such as pHluorins ( 366) that are sensitive to pH changes in the physiological range ...
Why are strip tests used?
Strips can be used to immobilize capture or reporter molecules to avoid any background quenching by the matrix. They have been employed for direct fluorescence measurement that do not involve FRET or quenching. Aptamers recognizing pathogens in food were used for their detection using lateral flow test strips that had capture antibodies ( Bruno, 2014 ). The reporters were aptamers labeled with either AuNPs or QDs, giving visible color or fluorescence signals.
What are the imaging agents that constitute the focus of our attention?
In this chapter, the imaging agents that constitute the focus of our attention are typically fluorescent molecules or radiolabeled bioconjugates. The majority of the fluorophores discussed possess NIR absorbance characteristics and the PET/SPECT capability generally tends to come from the modification of an immobilized molecule with copper, gallium, or indium in their radioactive isotope forms. Particular attention is paid to peptide sequences and their versatility as cancer targeting biomolecules.
What is the signaling agent used in imaging?
Presence of a suitable signaling agent to facilitate the imaging, such as a radioactive isotope, fluorescent molecule, or a combination of both.
What is a fluorophores?
Fluorophores are microscopic molecules, which may be proteins, small organic compounds, or synthetic polymers that absorb light of specific wavelengths and emit light of longer wavelengths. Certain semiconducting metallic nanoparticles also qualify as fluorophores, emitting light relative to their geometric composition.
What are the most commonly used fluorophores?
Soluble organic dyes are among the most commonly applied form of fluorophore for fluorescence microscopy applications. They can be conjugated with macromolecules to enable tracking and characterization of particles in vitro. Photostable Fluorescein, Rhodamine, and Cyanine are all acknowledged fluorophores for fluorescence microscopy and flow cytometry applications, absorbing wavelengths between 494 nm and emitting wavelengths of up to 633 nm.
How does fluorescence work?
In fluorescence microscopy, a fluorophore is chemically bonded to macromolecules and introduced to a sample dish or chamber. The incident light of the microscope can excite the entire sample or individual particles within the analyte to determine its fluorescent behavior. This process is complicated by the low energy output of fluorescent processes, as absorption and emission signals often overlap. Drawing any significant conclusions about a sample’s fluorescent characteristics, therefore, requires exceptionally discriminating bandpass filters to separate peak wavelength behaviors and make fluorescent signals detectable by the optical sensor or analyst.
What are the different types of fluorophores?
Types of Fluorophores. Fluorophores typically fall into one of the following categories: Biological fluorophores; Organic dyes; Quantum dots. Biological fluorophores are commonly comprised of fluorescent proteins such as GFP. They have been used for cell labeling and characterization with varied rates of success.
How weak is fluorescent microscopy?
Fluorescence microscopy is a method of observing photo-emissive spectra from samples, and acquiring fluorescent signals that are roughly one million times weaker than the incident or one thousand times weaker than the scattered light.
What happens to the excited state of a fluorophore?
During this time, the fluorophore undergoes conformational changes and is also subject to a multitude of possible interactions with its molecular environment. These processes have two important consequences. First, the energy of S 1 ' is partially dissipated, yielding a relaxed singlet excited state (S 1) from which fluorescence emission originates. Second, not all the molecules initially excited by absorption (Stage 1) return to the ground state (S 0) by fluorescence emission. Other processes such as collisional quenching, fluorescence resonance energy transfer (FRET) ( Fluorescence Resonance Energy Transfer (FRET)—Note 1.2) and intersystem crossing (see below) may also depopulate S 1. The fluorescence quantum yield, which is the ratio of the number of fluorescence photons emitted (Stage 3) to the number of photons absorbed (Stage 1), is a measure of the relative extent to which these processes occur.
What is the process of fluorescence?
Fluorescence is the result of a three-stage process that occurs in certain molecules (generally polyaromatic hydrocarbons or heterocycles) called fluorophores or fluorescent dyes ( Figure 1 ). A fluorescent probe is a fluorophore designed to respond to a specific stimulus or to localize within a specific region of a biological specimen. The process responsible for the fluorescence of fluorescent probes and other fluorophores is illustrated by the simple electronic-state diagram (Jablonski diagram) shown in Figure 2.
How to reduce photobleaching?
The most effective remedy for photobleaching is to maximize detection sensitivity, which allows the excitation intensity to be reduced. Detection sensitivity is enhanced by low-light detection devices such as CCD cameras, as well as by high–numerical aperture objectives and the widest bandpass emission filters compatible with satisfactory signal isolation. Alternatively, a less photolabile fluorophore may be substituted in the experiment. Molecular Probes™ Alexa Fluor 488 dye is an important fluorescein substitute that provides significantly greater photostability than fluorescein ( Figure 8, Figure 9, ), yet is compatible with standard fluorescein optical filters. Antifade reagents such as Molecular Probes™ SlowFade and ProLong reagents ( Fluorescence Microscopy Accessories and Reference Standards—Section 23.1) can also be applied to reduce photobleaching; however, they are usually incompatible with live cells. In general, it is difficult to predict the necessity for and effectiveness of such countermeasures because photobleaching rates are dependent to some extent on the fluorophore's environment.
How to enhance fluorescence?
The most straightforward way to enhance fluorescence signals is to increase the number of fluorophores available for detection. Fluorescent signals can be amplified using 1) avidin–biotin or antibody–hapten secondary detection techniques, 2) enzyme-labeled secondary detection reagents in conjunction with fluorogenic substrates or 3) probes that contain multiple fluorophores such as phycobiliproteins or FluoSpheres fluorescent microspheres. Our most sensitive reagents and methods for signal amplification are discussed in Ultrasensitive Detection Technology—Chapter 6.
What are the elements of fluorescence detection?
Four essential elements of fluorescence detection systems can be identified from the preceding discussion: 1) an excitation light source ( Figure 5), 2) a fluorophore, 3) wavelength filters to isolate emission photons from excitation photons ( Figure 5), 4) a detector that registers emission photons and produces a recordable output, usually as an electrical signal. Regardless of the application, compatibility of these four elements is essential for optimizing fluorescence detection.
Why are fluorescent reference standards important?
Because fluorescence quantitation is dependent on the instrument, fluorescent reference standards are essential for calibrating measurements made at different times or using different instrument configurations. To meet these requirements, we offer high-precision fluorescent microsphere reference standards for fluorescence microscopy and flow cytometry and a set of ready-made fluorescent standard solutions for spectrofluorometry ( Fluorescence Microscopy Accessories and Reference Standards—Section 23.1 , Flow Cytometry Reference Standards—Section 23.2 ).
How does binding affect fluorescence?
Binding of a probe to its target can dramatically affect its fluorescence quantum yield ( Monitoring Protein-Folding Processes with Environment- Sensitive Dyes—Note 9.1 ). Probes that have a high fluorescence quantum yield when bound to a particular target but are otherwise effectively nonfluorescent yield extremely low reagent background signals. The ultrasensitive SYBR, SYTO, PicoGreen, RiboGreen and OliGreen nucleic acid stains ( Nucleic Acid Detection and Analysis—Chapter 8) are prime examples of this strategy. Similarly, fluorogenic enzyme substrates, which are nonfluorescent or have only short-wavelength emission until they are converted to fluorescent products by enzymatic cleavage, allow sensitive detection of enzymatic activity ( Enzyme Substrates and Assays—Chapter 10 ).
What happens to energy during fluorescence?
And finally, we’re ready for some fluorescence! Beginning at the “relaxed” excited state, the high energy photon decays quickly toward the ground state and emits this excess energy as a photon of light. This transition of energy is what we know as fluorescence. Interestingly, because some of that energy was already released during the excited-state lifetime, the energy of the now fluorescing photon is lower than the energy of the excitation photon. Thus, the energy released during fluorescence will always be of a longer wavelength than that needed for excitation.
What Exactly IS Fluorescence?
By definition, fluorescence is a type of photoluminescence, which is what happens when a molecule is excited by ultraviolet or visible light photons. More specifically, fluorescence is the result of a molecule absorbing light at a specific wavelength and emitting light at a longer wavelength.
What is the most important tool in biology?
Fluorescence is one of the most important and useful tools in a biologist’s toolbox. In biology, nearly every field, from physiology to immunology, uses fluorescent molecules (aka fluorophores) to detect proteins. However, the specific science behind how fluorescence works can be confusing or overlooked.
What is the first step in absorbing light?
Step 1: Excitation . Flashback to General Chemistry: visible light exists as elementary particles called photons. These particles are essential packets of energy that when absorbed, will propel or “excite” the light-absorbing molecule into a higher energy level.
Is the energy of the fluorescing photon lower than the energy of the excitation photon?
Interestingly, because some of that energy was already released during the excited-state lifetime, the energy of the now fluorescing photon is lower than the energy of the excitation photon. Thus, the energy released during fluorescence will always be of a longer wavelength than that needed for excitation.
How do flow cytometers work?from ncbi.nlm.nih.gov
Flow cytometers utilize lasers as light sources to produce both scattered and fluorescent light signals that are read by detect ors such as photodiodes or photomultiplier tubes. These signals are converted into electronic signals that are analyzed by a computer and written to a standardized format (.fcs) data file.
How to measure fluorescence?from expert.cheekyscientist.com
In order to measure fluorescence from labeled cells, a light source is necessary to produce this fluorescence. Light can be generated in several ways, but the most effective way for the conditions and configurations of flow cytometry is by utilizing the laser.
What are fire dyes used for?from biocompare.com
“Our Fire™ Dyes are tandem fluorophores that are designed to fill spectral spaces previously unused in conventional flow cytometry,” explains Yamamoto. “Some of them have been specifically developed for spectral cytometry applications, such as APC/Fire™ 810, which is ideally suited to the latest spectral cytometers that have enhanced detection in the 800 nm range compared to older instruments. Our new PE/Fire™ 640 is another useful addition to spectral cytometry panels, where it can be combined with widely adopted fluorophores like PE/Dazzle™ 594 and PE/Cyanine5. We’re also actively growing our range of Spark Dyes; these small synthetic fluorophores have relatively narrow excitation and emission profiles and are stable from signal quenching by various standard fixation and permeabilization buffers.”
How many nanometers does eGFP emit?from augusta.edu
Green Fluorescent Protein (eGFP) can be excited at 488 nanometers with a peak emission at 509 nanometers and is detected in the FL1 detector on the FACSCalibur. The FACSCanto and LSRII and all of the resource's sorter flow cytometers are able to distinguish between concurrently expressing eGFP and eYFP cells when the proper optical filters and experimental controls exist. More detailed discussion of this molecule can be found in the references listed in the Protocols & Useful Links section of this Web site.
What is the purpose of lasers?from expert.cheekyscientist.com
Lasers illuminate the stream with coherent, focused light of specific wavelength (energy) and power. This illumination facilitates the generation of fluorescence signals from cells labeled with fluorophores and light scatter signal from redirected laser light.
How do tandem dyes work?from bio-rad-antibodies.com
Tandem dyes chemically couple either phycobiliproteins (PE, APC, PerCP) or polymers dyes (BV421, BUV395) with small organic fluorochromes (Cy3, Cy5, Cy7) to create a dye that uses fluorescence energy transfer (FRET) to increase the available fluorochromes that can be excited with a single laser source. For example, Texas Red has a maximum excitation of 589 nm, and PE has an emission of 585 nm, so by coupling PE to Texas Red, the emission from PE is used to excite Texas Red using FRET allowing PE-TxRed to be excited by either a 488 nm or 532 nm laser. The polymer chain antibodies use the same method to increase available fluorochromes that can be excited by a single laser. Tandem dyes are extremely bright with large Stokes shift values (150–300 nm) which is useful when dealing with low antigen density. However, tandem dyes are less stable than the donor fluorochromes and can differ from lot to lot in their energy transfer efficiency, complicating compensation. Most of the longer Brilliant polymer dyes are also tandems and share these issues.
What is FITC used for?from augusta.edu
Fluorescein isothiocyanate (FITC) is currently the most commonly used fluorescent dye for flow cytometry analysis. When excited at 488 nanometers, FITC has a green emission that is usually collected at 530 nanometers, the FL1 detector of a FACSCalibur. FITC has a high quantum yield (efficiency of energy transfer from absorption to emission fluorescence) and approximately half of the absorbed photons are emitted as fluorescent light. FITC is seldom used for fluorescent microscopy applications as it photobleaches rather quickly although in flow cytometry applications, its photobleaching effects are not observed due to a very brief interaction at the laser intercept. FITC is highly sensitive to pH extremes.
What does it mean to have a bright fluorophore?
Having a bright fluorophore means that you can use less excitation power and/or shorter exposures to collect the needed photons for your microscopy image.
What is the excitation and emission of a fluorophore?
It is imperative to understand that the excitation and emission for a fluorophore is not a single wavelength. For example, Alexa 488 is listed with an excitation max of 490 nm, and an emission max 526 nm. The reality is that there is greater than 10% efficiency to excite GFP from about 450 nm- 525 nm. This means that if you have high light levels, and sufficient fluorophore present, the excitation in that range will result in some amount of emission. This is why you can observe GFP signals with cubes designed for GFP or YFP (Figure 1). However, you will have a brighter signal with less illumination if the cube is matched well with your fluorophore.
What is the color of the eGFP vs YFP filter?
Figure 1. eGFP (A) vs YFP (B) filters with AlexaFluor 488 dye. Excitation (left curve) and emission right curve) of AlexaFluor 488 is displayed in green. The blue line indicates the wavelengths that are transmitted during excitation, while the red line indicates the wavelengths transmitted for emission. The black line is a dicrotic mirror to prevent transmission back through the incorrect filter. Both filter sets cover some of the excitation/emission curves of AlexaFluor 488, so you would be able to observe the fluorescence with either filter. Curves created with Chroma Spectra Viewer
What is bleedthrough in flow cytometry?
Bleedthrough or “cross talk” occurs when more than one fluorophore becomes excited and emits in the same channel. This can affect your quantification measurements as well as your image quality. Therefore, once you have set up your experiment, it is very important to run controls such as “fluorescent minus one” (FMO). You might not be gating, but the same concepts as used in flow cytometry still apply.
How long does it take for a protein to mature?
For example, eGFP requires 25 minutes to mature while mNeonGreen requires 10 minutes. The time to maturation can be over two hours ( mRuby3 )!
What are the labels on an Olympus filter?
In these filters from Olympus Microscopy, the labels are the yellow rectangles on the side of the filter.
Can you gating flow cytometry?
You might not be gating, but the same concepts as used in flow cytometry still apply. Now, before you get to your wet lab work, there are a few best practices to mitigate future problems. The first and easiest fix is to pick fluorophores that are as far away from each other on the light spectrum as possible.
Why are fluorescent proteins used in live cells?
In live cells, fluorescent proteins are most commonly employed to track the localization and dynamics of proteins, organelles, and other cellular compartments.
How to adapt fluorescent proteins?
In order to adapt fluorescent proteins for use in mammalian systems, several basic modification s of the wild-type green fluorescent protein were undertaken and are now found in all commonly used variants. The first step was to optimize the maturation of fluorescence to a 37-degree Celsius environment. Maturation of the wild-type fluorophore is quite efficient at 28 degrees, but increasing the temperature to 37 degrees substantially reduces overall maturation and results in decreased fluorescence. Mutation of the phenylalanine residue at position 64 ( Phe64) to leucine results in improved maturation of fluorescence at 37 degrees, which is at least equivalent to that observed at 28 degrees. This mutation is present in the most popular varieties of fluorescent proteins derived from Aequorea victoria, but is not the only mutation that improves folding at 37 degrees as other variants have been discovered.
How does denaturation affect fluorescent proteins?
Denaturation of green fluorescent protein destroys fluorescence, as might be expected, and mutations to residues surrounding the tripeptide fluorophore can dramatically alter the fluorescence properties. The packing of amino acid residues inside the beta barrel is extremely stable, which results in a very high fluorescence quantum yield (up to 80 percent). This tight protein structure also confers resistance to fluorescence variations due to fluctuations in pH, temperature, and denaturants such as urea. The high level of stability is generally altered in a negative manner by mutations in green fluorescent protein that perturb fluorescence, resulting in a reduction of quantum yield and greater environmental sensitivity. Although several of these defects can be overcome by additional mutations, derivative fluorescent proteins are generally more sensitive to the environment than the native species. These limitations should be seriously considered when designing experiments with genetic variants.
What are two examples of multiple fluorescent protein labeling in living cells?
The opossum kidney cortex proximal tubule epithelial cell ( OK line) presented in Figure 1 (a) was transfected with a cocktail of fluorescent protein variants fused to peptide signals that mediate transport to either the nucleus (enhanced cyan fluorescent protein; ECFP ), the mitochondria (DsRed fluorescent protein; DsRed2FP ), or the microtubule network (enhanced green fluorescent protein; EGFP ). A similar specimen consisting of human cervical adenocarcinoma epithelial cells ( HeLa line) is depicted in Figure 1 (b). The HeLa cells were co-transfected with sub-cellular localization vectors fused to cyan ( mTurquoise) and yellow ( mVenus) fluorescent protein coding sequences (Golgi complex and the nucleus, respectively), as well as the "Fruit" protein, mCherry, targeting the mitochondrial network.
How many nanometers does a green fluorescent protein absorb?
A protonated form, the predominant state, has an excitation maximum at 395 nanometers, and a less prevalent, unprotonated form that absorbs at approximately 475 nanometers.
What is the structure of fluorescent protein?
Although this simple amino acid motif is commonly found throughout nature, it does not generally result in fluorescence. What is unique to the fluorescent protein is that the location of this peptide triplet resides in the center of a remarkably stable barrel structure consisting of 11 beta -sheets folded into a tube.
What is the name of the protein that is found in jellyfish?
Osamu Shimomura and Frank Johnson, working at the Friday Harbor Laboratories of the University of Washington in 1961, first isolated a calcium-dependent bioluminescent protein from the Aequorea victoria jellyfish, which they named aequorin. During the isolation procedure, a second protein was observed that lacked the blue-emitting bioluminescent properties of aequorin , but was able to produce green fluorescence when illuminated with ultraviolet light. Due to this property, the protein was eventually christened with the unceremonious name of green fluorescent protein ( GFP ). Over the next two decades, researchers determined that aequorin and the green fluorescent protein work together in the light organs of the jellyfish to convert calcium-induced luminescent signals into the green fluorescence characteristic of the species.
What controls are required for each fluorochrome?from abcam.com
Compensation controls are required for each fluorochrome and should contain both a positive and a negative population. These controls should be solely used to set compensation. The positive should be at least as bright as anything that will be encountered in the experiment and should form at least 10% of the population.
How to set voltages for fluorescence channels?from abcam.com
Set voltages for fluorescence channels using an unstained sample. Adjust forward scatter and side scatter so that the cell population is clearly delineated. Dead cells, clumps and debris should be excluded from further analysis.
Where does the overlap occur in spectral emission?from abcam.com
Although most of the overlap will occur in the range on the higher wavelength side of the peak emission, due to the shape of the spectral emission curve, there may also be some bleed through/overlap to the opposite end, below the peak emission. This will become more of an issue as more fluorochromes are used.
Is PE-Cy5 a false positive signal?from abcam.com
This will be seen as "false positive" signals in the PE-Cy5 channel and fluorescence compensation is needed to correct for this overlap.
Can autofluorescence be used to stain a positive population?from abcam.com
The autofluorescence of the positive population, before staining, should be the same as the negative control. Ideally, the positive and negative populations in the control samples will be the same type of cell. If this is not possible, consider the use of compensation beads. Alternatively, different cells for your compensation controls can be used ...
Is fluorescence compensation the same as flow cytometer?from abcam.com
The procedure for setting correct fluorescence compensation is essentially the same on any cytometer but there are differences between the various available instruments, which makes it difficult to provide a "one size fits all" protocol. However, the following guidelines should be suitable in most cases. We always recommend reviewing the flow cytometer manufacturer's instructions for detailed compensation guidelines.
Overview
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds.
Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of …
Fluorescence
The fluorophore absorbs light energy of a specific wavelength and re-emits light at a longer wavelength. The absorbed wavelengths, energy transfer efficiency, and time before emission depend on both the fluorophore structure and its chemical environment, as the molecule in its excited state interacts with surrounding molecules. Wavelengths of maximum absorption (≈ excitation) and emission (for example, Absorption/Emission = 485 nm/517 nm) are the typical te…
Size (molecular weight)
Most fluorophores are organic small molecules of 20 - 100 atoms (200 - 1000 Dalton - the molecular weight may be higher depending on grafted modifications, and conjugated molecules), but there are also much larger natural fluorophores that are proteins: green fluorescent protein (GFP) is 27 kDa and several phycobiliproteins (PE, APC...) are ≈240kDa. In 2020, the smallest known fluorophore was claimed to be 3-hydroxyisonicotinaldehyde, a compound of 14 atoms and only 123 Da.
Families
Fluorophore molecules could be either utilized alone, or serve as a fluorescent motif of a functional system. Based on molecular complexity and synthetic methods, fluorophore molecules could be generally classified into four categories: proteins and peptides, small organic compounds, synthetic oligomers and polymers, and multi-component systems.
Applications
Fluorophores have particular importance in the field of biochemistry and protein studies, e.g., in immunofluorescence but also in cell analysis, e.g. immunohistochemistry and small molecule sensors.
Uses outside the life sciences
Additionally fluorescent dyes find a wide use in industry, going under the name of "neon colours", such as:
• Multi-ton scale usages in textile dyeing and optical brighteners in laundry detergents
• Advanced cosmetic formulations; safety equipment and clothing
See also
• Category:Fluorescent dyes
• Fluorescence in the life sciences
• Quenching of fluorescence
• Fluorescence recovery after photobleaching (FRAP) - an application for quantifying mobility of molecules in lipid bilayers.
External links
• The Database of fluorescent dyes
• Table of fluorochromes
• The Molecular Probes Handbook - a comprehensive resource for fluorescence technology and its applications.