
See more
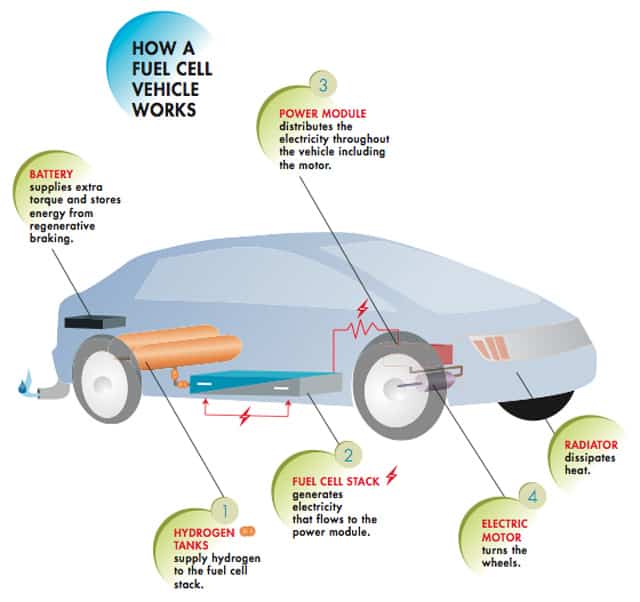
How does hydrogen fuel cells generate electricity?
Hydrogen fuel cells produce electricity by combining hydrogen and oxygen atoms. The hydrogen reacts with oxygen across an electrochemical cell similar to that of a battery to produce electricity, water, and small amounts of heat.
Can hydrogen be used to generate electricity?
Hydrogen can be used in fuel cells to generate electricity, or power and heat. Today, hydrogen is most commonly used in petroleum refining and fertilizer production, while transportation and utilities are emerging markets.
How much electricity does a hydrogen fuel cell produce?
approximately 1 voltA single fuel cell produces approximately 1 volt or less — barely enough electricity for even the smallest applications. To increase the amount of electricity generated, individual fuel cells are combined in series to form a stack.
Can you burn hydrogen to produce electricity?
Hydrogen as an Energy Carrier Because hydrogen does not exist freely in nature and is only produced from other sources of energy, it is known as an energy carrier. It is a clean-burning fuel, and when combined with oxygen in a fuel cell, hydrogen produces heat and electricity with only water vapor as a by-product.
Why don't we use hydrogen for power?
We don't yet have a hydrogen economy because: Elemental hydrogen is scarce. The cheapest way to make hydrogen also makes lots of carbon dioxide. Carbon dioxide emissions are not yet priced.
Why do we not use hydrogen power?
Safety concerns. The need for onboard hydrogen fuel tanks has created safety concerns and limited the uptake of hydrogen fuel cell electric vehicles (FCEVs). Auto manufacturers like Toyota have been quick to rebuke these concerns, maintaining that FCEVs are just as safe as their petrol and diesel-powered counterparts.
Why are hydrogen fuel cells inefficient?
Hydrogen's efficiency problem The reason why hydrogen is inefficient is because the energy must move from wire to gas to wire in order to power a car. This is sometimes called the energy vector transition.
Is hydrogen better than electric cars?
As with electric cars, hydrogen cars produce zero harmful emissions on the road, but unlike electric cars, which take a fair amount of time to recharge, hydrogen cars can be refilled about as quickly as a petrol or diesel car.
What is more efficient hydrogen or electric cars?
However, as hydrogen cars densely pack their energy storage, they're usually able to achieve longer distances. While most fully electric vehicles can travel between 100-200 miles on a single charge, hydrogen ones can get to 300 miles, according to AutomotiveTechnologies.
What are some of the downsides of producing hydrogen fuel?
What are the Disadvantages of Hydrogen Fuel Cells?Hydrogen Extraction. ... Investment is Required. ... Cost of Raw Materials. ... Regulatory Issues. ... Overall Cost. ... Hydrogen Storage. ... Infrastructure. ... Highly Flammable.
What is the obstacle to using hydrogen as a fuel?
The key challenges include: Weight and Volume. The weight and volume of hydrogen storage systems are presently too high, resulting in inadequate vehicle range compared to conventional petroleum fueled vehicles.
What is left after you burn hydrogen?
The most important benefit of using hydrogen as a fuel is that when you burn it, the byproduct is just water. Hydrogen can also be used as a way to store energy, and this use has the potential to have a large impact on our future.
Can you run a turbine on hydrogen?
Hydrogen–gas turbines have many environmental and economic benefits, and Mitsubishi Power is committed to facilitating the transition. Their turbines can be fitted into existing power plants and can run on less pure forms of hydrogen, which can be carried in any form, from liquid hydrogen to ammonia.
Can a turbine engine run on hydrogen?
Liquid or gaseous hydrogen is burned in a gas-turbine engine to generate thrust. Combustion is a chemical process in which energy is released from a mixture of fuel and air. Proponents of hydrogen say that its wide flammability range and high-auto ignition temperature make it particularly suitable for combustion.
Can you run a motor on hydrogen?
Both hydrogen internal combustion engines and hydrogen fuel cells can power vehicles using hydrogen, a zero-carbon fuel. Hydrogen engines burn hydrogen in an internal combustion engine, in just the same way gasoline is used in an engine.
What are the pros and cons of hydrogen power?
Hydrogen fuel cells Pros: No vehicle emissions other than water vapor. Fuel economy equivalent to about twice that of gasoline vehicles. Hydrogen is abundant, and can be made from renewable energy. Cons: This space-age technology is expensive.
What is hydrogen fuel cell?
Hydrogen is the simplest and most abundant element in the universe. It is a major component of water, oil, natural gas, and all living matter . Despite its simplicity and abundance, hydrogen ...
How is hydrogen produced?
Once hydrogen is produced as molecular hydrogen, the energy present within the molecule can be released, by reacting with oxygen to produce water.
What is hydrogen energy?
Hydrogen, therefore, is an energy carrier, which is used to move, store, and deliver energy produced from other sources. Learn more about: Hydrogen fuel. Fuel cells.
How does hydrogen gas enter a fuel cell?
Pressurised hydrogen gas enters the fuel cell on the anode side. The pressure forces the gas through the catalyst (commonly platinum) which causes the hydrogen gas to split into two H+ ions and two electrons (e-). The electrons are conducted through the anode where they make their way through an external circuit (where they can power things) and return to the cathode side of the fuel cell. The anode has channels that disperse the hydrogen gas equally over the surface of the catalyst.
Where do electrons go in a fuel cell?
The electrons are conducted through the anode where they make their way through an external circuit (where they can power things) and return to the cathode side of the fuel cell. The anode has channels that disperse the hydrogen gas equally over the surface of the catalyst.
What causes oxygen gas to split?
Meanwhile on the cathode side of the fuel cell, pressurised oxygen gas (O2) is forced through the catalyst which causes the oxygen gas to split into two oxygen atoms (O). Each of these atoms has a strong negative charge. The cathode has channels that distribute the oxygen to the surface of the catalyst.
What is the reaction that produces energy and converts it into electricity?
The strong negative charge of the Oxygen atoms attracts the H+ ions through the membrane where they combine with an Oxygen atom and two of the electrons from the external circuit to form a water molecule (H2O). This reaction produces energy which is captured and converted to electricity.
What is the most promising system to power cars and other transport?
Put simply, a hydrogen fuel cell converts hydrogen gas and oxygen gas into water and, in the process, produces electricity. The Proton Exchange Membrane Fuel Cell ( PEMFC) is considered the most promising system to power cars and other transport.
How are the anode and cathode separated?
The anode and the cathode are separated by an electrolyte membrane. The membrane is a specially treated material that conducts only positively charged ions (that is, it allows positively charged ions to pass through it and blocks electrons from passing through it).
What can hydrogen fuel cells provide?
Photo of two hydrogen fuel cells. Fuel cells can provide heat and electricity for buildings and electrical power for vehicles and electronic devices.
What is the purpose of alkaline fuel cells?
Alkaline fuel cells use an alkaline electrolyte such as potassium hydroxide or an alkaline membrane that conducts hydroxide ions rather than protons. Originally used by the National Aeronautics and Space Administration (NASA) on space missions, alkaline fuel cells are now finding new applications, such as in portable power.
What is phosphoric acid fuel cell?
Phosphoric acid fuel cells use a phosphoric acid electrolyte that conducts protons held inside a porous matrix, and operate at about 200°C. They are typically used in modules of 400 kW or greater and are being used for stationary power production in hotels, hospitals, grocery stores, and office buildings, where waste heat can also be used. Phosphoric acid can also be immobilized in polymer membranes, and fuel cells using these membranes are of interest for a variety of stationary power applications.
What is a direct methanol fuel cell?
The direct-methanol fuel cell (DMFC) is similar to the PEM cell in that it uses a proton conducting polymer membrane as an electrolyte. However, DMFCs use methanol directly on the anode, which eliminates the need for a fuel reformer.
What is the difference between a fuel cell and a polymer electrolyte membrane?
A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode)—sandwiched around an electrolyte. A fuel, such as hydrogen, is fed to the anode, and air is fed to the cathode. In a polymer electrolyte membrane fuel cell, a catalyst separates hydrogen atoms into protons and electrons, ...
What is a PEM fuel cell?
Polymer electrolyte membrane (PEM) fuel cells, also called proton exchange membrane fuel cells, use a proton-conducting polymer membrane as the electrolyte. Hydrogen is typically used as the fuel. These cells operate at relatively low temperatures and can quickly vary their output to meet shifting power demands.
What is molten carbonate fuel cell?
Molten Carbonate Fuel Cells. Molten carbonate fuel cells use a molten carbonate salt immobilized in a porous matrix that conducts carbonate ions as their electrolyte. They are already being used in a variety of medium-to-large-scale stationary applications, where their high efficiency produces net energy savings.
How does hydrogen fuel cell work?
In a hydrogen fuel cell, the catalyst at the anode will separate hydrogen molecules into electrons and protons, which will then take different paths to the cathode. The electrons then go through an external circuit, which in turn creates a flow of electricity.
How does hydrogen produce electricity?
How can Hydrogen Generate Electricity. Hydrogen fuel cells will produce electricity by combining oxygen and hydrogen atoms. The hydrogen will react with oxygen across an electrochemical cell similar to a battery to produce water, small amounts of heat and electricity. Several different types of fuel cells are available for a wide range ...
Where is Hydrogen Found?
Hydrogen is found in most of the stars and the sun. The planet Jupiter is composed of mainly hydrogen.
How to use hydrogen in a car?
One of the most useful ways to use hydrogen is in electric buses or cars alongside a fuel cell which will convert the hydrogen into electricity. Fuel cells are desirable due to being far more efficient than an internal combustion engine it can replace, although the latter can still be used with hydrogen fuels if desired.
What is hydrogen used for?
Hydrogen is used to create electrical power in fuel cells . Hydrogen can be used as the fuel in internal combustion engines to replace diesel or petrol. Pound for pound it actually contains more than three times the energy of the majority of most hydrocarbon fuels. It is non-toxic, odourless and invisible.
What is the future of hydrogen fuel cells?
What is the Future of Hydrogen Fuel Cell? Our cars, in the not too distant future, could be powered by Hydrogen fuel cells. Hydrogen would replace the petroleum fuel that is used in the majority of today's vehicles. Many vehicle manufacturers are actively developing and researching transportation hydrogen fuel cell technologies.
What is hydrogen economy?
The term Hydrogen Economy refers to a vision for the use of hydrogen as an external carbon energy source. It would replace gasoline and diesel as a fuel for transportation or as a natural gas to be used as heating fuel. In all cases, it will take energy to convert these into pure hydrogen.
How do fuel cells generate electricity?
The negatively-charged electrons go through an external circuit, where they generate electricity. The protons migrate through the electrolyte to the cathode. Once the electrons complete their journey, they reunite with the protons, and combine with the oxygen to produce water (and heat). Fuel cells generate direct current (DC) ...
What are Hydrogen Fuel Cells?
Hydrogen fuel cells produce electricity in a similar way that lithium-ion battery cells power electric cars – but Hydrogen fuel cells do not run down or need recharging.
Where is hydrogen stored in a fuel cell?
Hydrogen (stored in tanks or reformers) is fed using Sundyne PPI compressors to the anode side of the fuel cell, while oxygen (from the air) is fed to the cathode side. On the anode side, a catalyst (typically platinum) splits hydrogen molecules into protons and electrons.
How many volts does a fuel cell produce?
In most cases, a single fuel cell produces less than 1 Volt – so individual cells are combined into stacks consisting of hundreds of fuel cells. Fuel cell performance improves as the pressure of the reactant gases increases; therefore fuel cell systems require compressors to raise inlet pressure several times greater than the pressure ...
