
If the last digit of the DEA number matches the last digit of the number obtained in step 5, the DEA number is considered valid. The last digit of BB1388568 and 48 is “8”. This checksum indicates that the DEA number is valid.
How do you verify a DEA?
How do you verify a DEA?
- Add together the first, third, and fifth digits.
- Add together the second, fourth, and sixth digits. Multiply the sum by 2.
- Add together the totals from Step 1 and Step 2.
- Verify that the last digit of the result of Step 3 matches the check digit of the DEA number.
How to verify a DEA?
Here are the steps to verify a DEA number: Step 1: add the first, third, and fifth digits of the DEA number. Step 2: add the second, fourth, and sixth digits of the DEA number. Step 3: multiply the result of Step 2 by two. Also, what do the letters in DEA number represent?
How do you calculate DEA?
To calculate the deductions from your employee’s pay you’ll have to:
- work out the employee’s earnings after tax, class 1 National Insurance and workplace pension contributions
- deduct the percentage shown in the table from the employee’s earnings
- check if the employee has other debt orders and if they take priority over Direct Earnings Attachment ( DEA) - see the employer’s guide
How to calculate the DEA number?
multiply the net earnings figure by the percentage rate – Standard or Higher – to calculate the DEA amount Note: If you are calculating a DEA based on a daily rate, you must also multiply the daily rate figure by the number of days in the pay period. (please refer to the more detailed guide for employers at 7.12 a) and b))
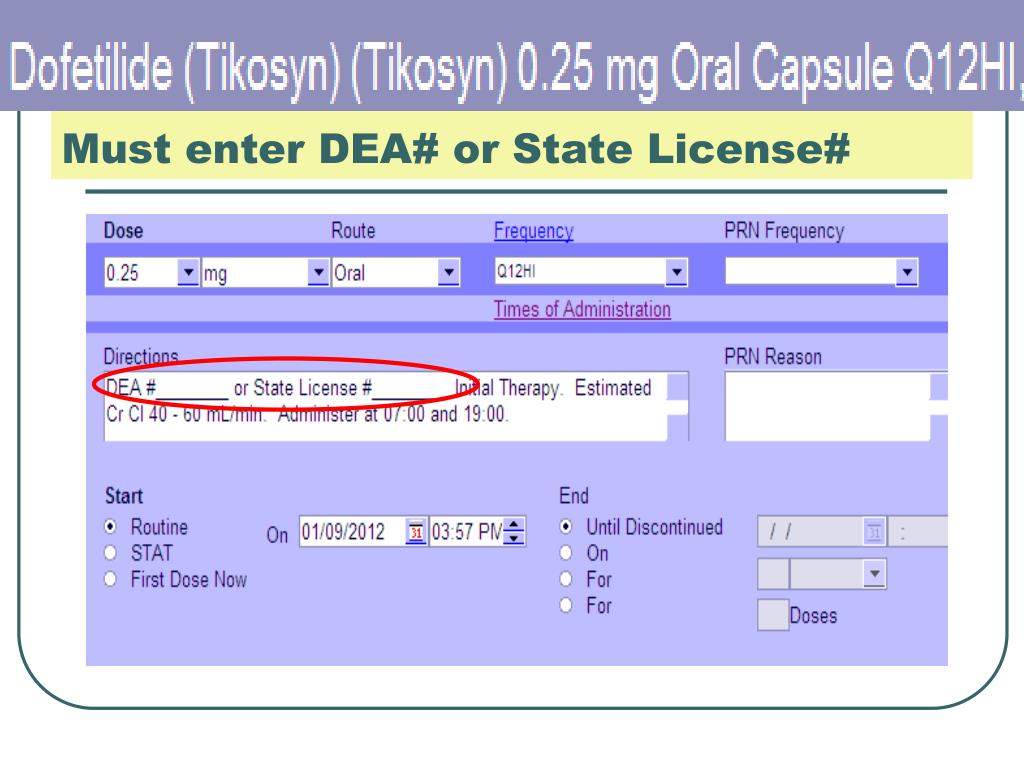
How do I make sure my DEA is valid?
Drug Enforcement Agency assigns authorized practitioners and hospitals a unique number in order to keep track of the distribution of controlled drugs. The instructions below explain how a DEA number verification is done. DEA numbers can be verified by using the last number, which is known as the Check Digit.
What is the check digit in a DEA number?
DEA numbers follow a specific format — two letters, six numbers, and one check digit — that verifies the number's validity and reveals the prescriber.
What letters do DEA numbers start with?
DEA is announcing that, effective immediately, DOD personal service contractors will be issued a new DEA registration number that begins with the letter "G". This new first character will be in addition to the current first characters A, B, F of the DEA registration for practitioners.
Why does it matter if a DEA number is invalid?
Why is this DEA number invalid? The last number should be 6. known as the "verification number." It is not valid, because the final digit should be 9.
What is a valid DEA?
Current format. A valid DEA number consists of: 2 letters, 6 numbers, and 1 check digit. The first letter is a code identifying the type of registrant (see below) The second letter is the first letter of the registrant's last name, or "9" for registrants using a business address instead of name.
What is the first letter of a DEA number mean?
The first letter is a code to identify the type of prescriber (i.e., a hospital, a practitioner, a manufacturer, etc.). The second letter is the first letter of the prescriber's last name. The seven numbers follow, and the seventh is the check digit. Let's walk though the steps of how to verify the DEA number. Dr.
What does B mean in a DEA number?
Hospital/ClinicB = Hospital/Clinic. C = Practitioner (i.e., a physician, dentist, veterinarian) D = Teaching Institution.
What is the letter A for in a DEA number?
DEA numbers consists of 2 letters, 6 digits, and 1 check digit. The first letter is a code identifying the type of registrant. (Usually you see A, B or C) A has been retired <1985,>B is used for Doctors and Clinics, C is for Specialists. The second letter is the first letter of the registrant's (prescriber) last name.
What is DEA suffix number?
An Institutional DEA Registration Number is a unique number issued by the U.S. Drug Enforcement Administration (“DEA”) to a licensed, eligible institution that handles controlled substances.
What does F mean in a DEA number?
DEA has stated that the first letter of the registration number is “almost always followed by the first letter of the registrant's last name” (for example, “F” for Dr. William Feelgood), then a computer-generated sequence of seven numbers (for example AF1234567).
Do I need a DEA license for each location in the same state?
DEA regulations require a separate registration for each principal place of business or professional practice at one general physical location where controlled substances are manufactured, distributed, imported, exported, or dispensed by a person. 21 CFR 1301.12(a).
Do you need DEA to prescribe antibiotics?
Under federal law, a DEA number is not technically required to write prescriptions for non-controlled medications such as antibiotics. Although a DEA number is not mandatory for medical providers who do not plan to prescribe controlled substances, practicing without one can cause a lot of headaches.
What does F mean in a DEA number?
DEA has stated that the first letter of the registration number is “almost always followed by the first letter of the registrant's last name” (for example, “F” for Dr. William Feelgood), then a computer-generated sequence of seven numbers (for example AF1234567).
What is a DAW code in pharmacy?
To answer this question, let's first understand what a Dispense as Written (DAW) code is. A DAW code specifies the prescriber's instructions to the payer regarding substitution of a generic equivalent or to dispense the specific prescribed medication.
Which is considered a mid level prescriber?
Examples of mid-level practitioners include, but are not limited to, health care providers such as nurse practitioners, nurse midwives, nurse anesthetists, clinical nurse specialists and physician assistants who are authorized to dispense controlled substances by the state in which they practice.
What would be the most appropriate way to handle a DEA Form 222 according to federal law?
Question: Who can sign executed DEA 222 Order Forms? Answer: Registrants, and individuals given power of attorney by registrants, can sign DEA 222 order forms. Any registrant may authorize one or more individuals to obtain and execute DEA Forms 222 by granting a power of attorney to each such individual.
How to check if a physician has a DEA number?
While the DEA does not give out registration information to the public, individuals are able to check whether or not a physician has a DEA number by contacting the state medical licensing board, advises the U.S. Drug Enforcement Administration.
What is a DEA certificate?
The DEA certificate displays the date the registration certificate was issued and its expiration date. The authenticity of a DEA registration certificate is verifiable by obtaining a list of current DEA license holders, notes the Office of Diversion Control.
How often does a DEA license expire?
DEA licensing expires every three years and must be renewed. Medical professionals are required to have a separate DEA registration certificate for every physical location controlled substances are dispensed, and physicians must only dispense drugs authorized by the state.
Can a DEA license number be verified?
A Drug Enforcement Administration license number cannot be verified, but requesting to see a copy of the official DEA registration certificate provides satisfactory proof of licensing, according to the DEA's Office of Diversion Control.
Can a DEA prescribe drugs?
DEA-registered medical practitioners are approved to prescribe and dispense controlled substance medications to patients, but practitioners can authorize an agent to dispense substances under strict limitations, reports National Association of Boards of Pharmacy.
When will the DEA send renewal notifications?
Instead, an electronic reminder to renew will be sent at 60, 45, 30, 15, and 5 days prior to the expiration date of the registration to the associated email address.
How long after a list 1 expired can you use a controlled substance?
Regardless of whether a registration is reinstated within the calendar month after expiration, federal law prohibits the handling of controlled substances or List 1 chemicals for any period of time under an expired registration.
Can a license renewal be extended beyond the expiration date?
If a renewal application is submitted in a timely manner prior to expiration, the registrant may continue operations, authorized by the registration, beyond the expiration date until final action is taken on the application.
How to look up DEA number?
You can look up a DEA number by using the practitioner’s name or their National Provider Identifier (NPI), a 10-digit number that is used to classify health care partners, including all payers. After every three years the DEA registration must be renewed.
What is a DEA number?
A DEA number is a unique identifier that enables medical practitioners to write controlled drug prescriptions. Two letters, six digits, and one check digit make up DEA numbers. DEA numbers are intelligent numbers, which means that some of the digits are meaningful. The first letter denotes the category of registrant:
What is the Controlled Substances Act?
The Controlled Substances Act requires a separate registration at each principal place of business or professional practice where the controlled substances are distributed or dispensed. See 21 U.S.C. 822(e)(1), 21 CFR 1301.12(a). Therefore, a practitioner who maintains a professional practice location in multiple states has established, ...
Does DEA require education?
Answer: No. DEA does not have any educational requirements, but individual states may have educational requirements in order to obtain and maintain a valid license in that state . DEA merely requires that all state licensing requirements be met in order to obtain a DEA registration in that state. 21 U.S.C. 823 (f) (1) and (4). EO-DEA182, October 5, 2020
Do you need a separate registration for a DEA?
DEA regulations require a separate registration for each principal place of business or professional practice at one general physical location where controlled substances are manufactured, distributed, imported, exported, or dispensed by a person. 21 CFR 1301.12 (a). DEA regulations do not prohibit individual and mid-level practitioners from using their home address.
Can a DEA prescribe controlled substances?
Answer: Yes. Since DEA's authority to register practitioners to dispense (including to prescribe) controlled substances is contingent, in part, upon the applicant's authorization in the state in which he or she practices, his or her controlled substance privileges and limits are determined by that specific state. The Controlled Substances Act requires a separate registration at each principal place of business or professional practice where the controlled substances are distributed or dispensed. See 21 U.S.C. 822 (e) (1), 21 CFR 1301.12 (a). Therefore, a practitioner who maintains a professional practice location in multiple states has established, for registration purposes, a principal place of business in each of those states. Consequently, DEA requires that the practitioner obtain a separate DEA registration in each state. Further, to do so the practitioner must first obtain authorization to handle controlled substances in each state where he or she has an office. For additional information please see the Final Rule titled: Clarification of Registration Requirements for Individual Practitioners, which DEA published in the Federal Register on December 1, 2006. EO-DEA181, November 2, 2020
