See more

What triggers the differentiation of monocytes into macrophages?
Growth factors, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and M-CSF play a principal role in their activation: GM-CSF drives the differentiation of “pro-inflammatory” monocytes to M1 macrophages, while M-CSF regulates differentiation of the “anti-inflammatory” subset of monocytes to M0 ...
How do monocytes become macrophages in atherosclerosis?
Monocyte-Derived Macrophages In atherosclerosis, plaque macrophages are largely derived from circulating monocytes infiltrating the plaque. Endothelial activation induces the arrest of monocytes onto the vessel wall where they transmigrate into the arterial wall, maturing into macrophages.
How do macrophages develop?
Macrophages are formed through the differentiation of monocytes, one of the major groups of white blood cells of the immune system. When there is tissue damage or infection, the monocytes leave the bloodstream and enter the affected tissue or organ and undergo a series of changes to become macrophages.
Are all macrophages derived from monocytes?
Originally described as part of the mononuclear phagocyte system, macrophages were long thought to derive solely from adult blood circulating monocytes. However, accumulating evidence now shows that certain macrophage populations are in fact independent from monocyte and even from adult bone marrow hematopoiesis.
How and when do monocytes and macrophages work towards innate immunity?
Monocytes/macrophages are critical for innate immunity, protecting us from self and non-self antigens. Homeostatic control of monocytes/macrophages is shared between pro- and anti-inflammatory molecules and is essential for the regulation of the immune system.
What does a macrophage become once it has ingested cholesterol?
The macrophages engulf oxidized low-density lipoproteins (LDLs) by endocytosis via scavenger receptors, which are distinct from LDL receptors. The oxidized LDL accumulates in the macrophages and other phagocytes, which are then known as foam cells.
Where are macrophages produced?
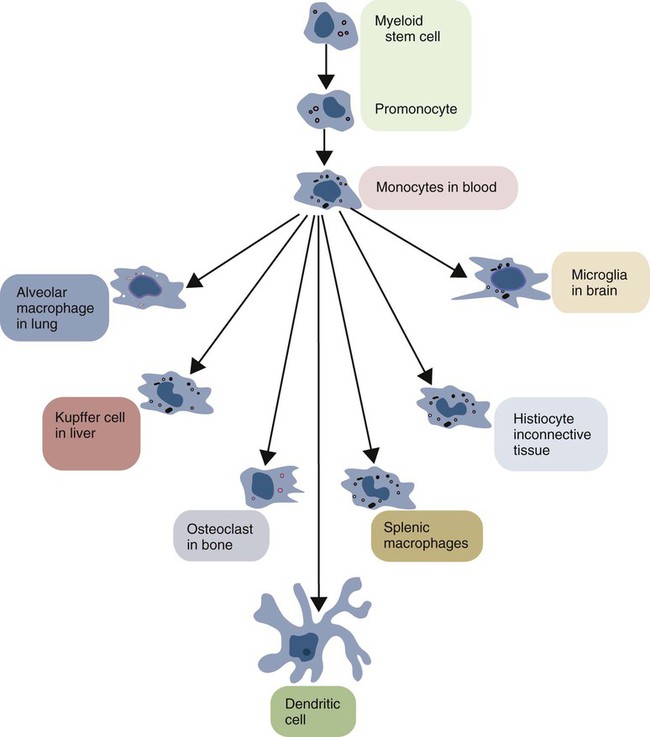
the bone marrowMacrophages develop in the bone marrow from cells known as monocytes. Monocytes arise from precursor cells under the influence of the granulocyte-macrophage colony-stimulating factor. They then leave the bone marrow and circulate in the blood.
How do monocytes differ from macrophages in vitro?
Macrophage Differentiation ProtocolIsolate mononuclear cells (day 0). ... Analyze mononuclear cells (day 0). ... Let the monocytes attach (day 0). ... Prepare the complete Macrophage Generation Medium DXF (day 0). ... Wash the adherent cell fraction (day 0). ... Start the macrophage differentiation (day 0).More items...
Which white blood cells are macrophages?
A type of immune cell that is made in the bone marrow and travels through the blood to tissues in the body where it becomes a macrophage or a dendritic cell. Macrophages surround and kill microorganisms, ingest foreign material, remove dead cells, and boost immune responses.
What do monocytes mature into?
In tissues, monocytes develop into much larger phagocytic cells known as macrophages.
How do monocytes become dendritic cells?
Monocytes differentiate into dendritic cells under inflammatory conditions in peripheral tissues. However, as early as the 1990s, Sallusto and Lanzavecchia15 and Romani et al. demonstrated that human monocytes differentiate into DCs in vitro by culturing with GM-CSF and IL-4.
Do non classical monocytes differentiate into macrophages?
From these studies we conclude that classical monocytes are the principal source of mo-DCs, but all subsets can differentiate to macrophages. We also found that monocytes, in particular the non-classical subset, represent an alternate source of type I IFN secretion in response to virus-associated TLR agonists.
Are monocytes?
What are monocytes? Monocytes are a type of white blood cell (leukocytes) that reside in your blood and tissues to find and destroy germs (viruses, bacteria, fungi and protozoa) and eliminate infected cells. Monocytes call on other white blood cells to help treat injury and prevent infection.
What is atheroma biology?
An atheroma (plaque) is a fatty material that builds up inside your arteries. It's made of cholesterol, proteins and other substances that circulate in your blood. Atheromas grow over time and may lead to coronary artery disease, peripheral artery disease, heart attack or stroke.
Where are monocyte-derived macrophages found?
Replenishment of monocyte-derived macrophages in the tissue (gut, skin, heart, and lung)
What are classical monocytes recruited to?
The original concept of MPS implicated that classical monocytes are recruited in the tissue to become tissue-resident macrophages in homeostatic conditions, and inflammatory activated macrophages during an infection (27, 54). We will examine more in detail the role of recruited cells during the inflammatory response later, while here we will focus on the recruitment of monocytes in homeostasis and their contribution to maintaining the pool of tissue macrophages. In order to avoid misunderstandings, it is important to agree on the definition of monocyte. In our view, bona fidemonocytes are restricted to the blood compartment, and to the bone marrow and spleen (55), where they wait to be released in the blood. For obvious reasons, in both these compartments, monocytes should not initiate any inflammatory reaction, but they must be ready to be recruited into the blood first and subsequently to all organs and tissues. A phenomenon was recently reported, termed “anticipatory inflammation,” whereby Ly6C+classical monocytes are released from the bone marrow in diurnal rhythmic waves under the control of circadian gene Bmal1(or Arntl) (56) to provide an adequate innate response to environmental challenges that are expected to occur with a evolutionarily predicted frequency. Despite new evidence supports the view that Ly6C+classical monocytes are not precursors of resident macrophages in all tissues and during certain types of inflammation (see below), it is clear that circulating monocytes contribute to the repopulation of tissue-resident macrophages under homeostatic conditions in tissues like the lamina propriaof the small intestine and healthy skin. Studies based on functional and lineage tracing and adoptive transfer have revealed that Ly6C+monocytes are precursors of intestinal macrophages that have a short half-life of only 3 weeks (57–59). Conversely, in the dermis are present both resident dermal macrophages and monocyte-derived macrophages (60, 61). A recent work suggests that the number of macrophages is partially replenished by monocytes also in the heart (62) and in the lung (63). It is unknown why some tissue macrophages are constantly maintained by circulating monocytes, whereas other populations are independent on circulating monocytes (see below). The notion that monocyte-derived macrophages derive from Ly6C+cells suggests that the repopulation/maintenance of resident macrophages in steady-state conditions follows the same mechanism as that occurring during inflammation.
What are the roles of monocytes in vivo?
The physiological role of the monocyte subsets in vivois not fully defined. They might have different roles during the homeostasis, immune defense/inflammation, and tissue repair, in terms of their capacity to become activated and secrete inflammatory cytokines in response to different stimuli, antigen processing and presentation, pro-angiogenic and patrolling behavior. The phenotypic and functional differences between the monocyte subsets were recently discussed in an exhaustive review (41). The authors of this review report a complete and referenced list of studies on bacterial and viral infections, autoimmune diseases, and inflammatory conditions, in which an expansion of CD16+cells in respect to other subsets has been observed. In general terms, both human classical and intermediate monocytes have inflammatory properties reminiscent of the murine Ly6C+monocytes (also termed “inflammatory” monocytes) (42), while non-classical monocytes display patrolling properties similar to those of murine Ly6C−monocytes (also termed “alternative” or “patrolling” monocytes) (43). Both human and mouse inflammatory monocytes express high levels of the chemokine receptor CCR2 and low levels of the chemokine receptor CX3CR1, whereas patrolling monocytes show a reverse pattern. Accordingly, inflammatory monocytes respond to the chemokine CCL2 that mediates Ly6C+/CD14+monocyte recruitment to inflammatory sites (44), while patrolling monocytes respond to CX3C-chemokine ligand 1 [CX3CL1, the human fractalkine and mouse neurotactin; (45)], a chemokine present both as soluble protein and as membrane-bound chemokine form that is expressed on endothelial cells and in tissues. Overall, it is clear that the subsets between human being and mouse are similar but not identical (42, 46). Table Table11summarizes the main features of monocytes in human beings and mice. Of note, there is a clear difference in the proportion of the two monocyte subsets, as Ly6C−cells represent about half of the circulating monocytes in mice, whereas CD16+monocytes account for less than 15% in human beings (30). However, Ziegler-Heitbrock hypothesized that the higher proportion of the Ly6C−in mouse blood could be due to stressful blood drawing (cardiac puncture under terminal anesthesia) that mobilizes these monocytes from the marginal pool (46). This hypothesis still needs experimental proof.
What is the homeostatic control of macrophages?
Homeostatic control of monocyte/macrophage development is mostly influenced by CSF-1 (also known as M-CSF), produced by stromal cells within the blood and in tissues (20). Mature mononuclear phagocytes in turn express CSF-1 receptors (CSF-1R) and remove circulating CSF-1, allowing a feedback loop responsible for monocyte proliferation decrease (21, 22). Recently, the cytokine IL-34 has been identified as able to bind and signal through the CSF-1R (6, 23). Unlike broadly expressed CSF-1, IL-34 expression is restricted to the epidermis and central nervous system (24), where it supports the steady-state proliferation of macrophages (LC and microglia, respectively). Granulocyte-macrophage colony-stimulating factor (GM-CSF) is another factor involved in the development of mononuclear phagocytes but only during the inflammatory state and not under homeostatic conditions (25, 26).
What is the role of macrophages in the immune system?
This highlights the central role of macrophages in immune defense, overturning the long-held notion that macrophages need to be activated by T-cells (14).
What are the roles of blood monocytes in inflammation?
The traditional view of the MPS suggests that recruited monocytes (that become macrophages in tissues) are key players during inflammation and pathogen challenge, whereas tissue-resident macrophages have important roles in development, tissue homeostasis, and the resolution of inflammation . A basic concept of the MPS is that blood monocytes are precursors that replace tissue macrophages within a single developmental lineage (4). This dogma needs now to be revised in the light of new evidence that macrophages are endowed with self-renewal capacity and can populate tissues before birth, deriving from early hematopoiesis in the yolk sac (12, 13). The discovery of new macrophage progenitors of embryonic origin forces us to reassess definitions, functions, and cell–cell relationships within the MPS. We can synthesize it in three key new questions:
What is the function of Ly6C+monocytes?
The function of Ly6C+monocytes in circulation remains poorly defined. In the attempt to identify an effective role of monocytes in the blood in homeostatic conditions (besides being precursor cells), a recent work has suggested a distinct surveillance phenotype for Ly6C+monocytes (64). These monocytes can enter non-lymphoid organs without obligatory differentiation into macrophages or DC. The authors propose that these monocytes can upregulate MHC class II expression and subsequently recirculate to lymph nodes, where they are able to present antigens to T-cells. Considering that these cells retain a monocyte-like gene expression profile, the authors term them “tissue monocytes” (64). This study contributes to revising the role of circulating monocytes, suggesting that they are not only precursors of macrophages but also effector cells.
Where do monocytes circulate?
Monocytes(Fig. 1A) circulate in the blood, bone marrow, and spleen and do not proliferate in a steady state (3, 4). Monocytes represent immune effector cells, equipped with chemokine receptors and pathogen recognition receptors that mediate migration from blood to tissues during infection. They produce inflammatory cytokines and take up cells and toxic molecules. They can also differentiate into inflammatory DCs or macrophages during inflammation, and possibly, less efficiently, in the steady state. Migration to tissues and differentiation to inflammatory DC and macrophages is likely determined by the inflammatory milieu and pathogen associated pattern recognition receptors (5).
What is the role of macrophages in lymphoid tissue?
Macrophages(Fig. 1, A and B) are resident phagocytic cells in lymphoid and non-lymphoid tissues, and are believed to be involved in steady-state tissue homeostasis via the clearance of apoptotic cells, and the production of growth factors. Macrophages are equipped with a broad range of pathogen recognition receptors that make them efficient at phagocytosis and induce production of inflammatory cytokines (6). The developmental origin and the function of tissue macrophage subsets, such as microglia (macrophages in the central nervous system), dermal macrophages (Fig. 1A), and splenic marginal zone and metallophilic macrophages (Fig. 1 B), remain insufficiently understood.
What are the major effectors of the immune system?
Monocytes and macrophages are critical effectors and regulators of inflammation and the innate immune response, the immediate, pre-programmed arm of the immune system. Dendritic cells initiate and regulate the highly pathogen-specific adaptive immune responses, and are central to the development of immunologic memory and tolerance. Recent in vivoexperimental approaches in the mouse have unveiled new aspects of the developmental and lineage relationships among these cell populations. Despite this, the origin and differentiation cues for many tissue macrophages, monocytes, and dendritic cell subsets in mice, and the corresponding cell populations in humans, remain to be elucidated.
What are the cytokines that control the development of phagocytes?
The development of the mononuclear phagocyte system is controlled by cytokines - small secreted proteins that promote cell-cell communication and can act as growth and differentiation factors. The generation of monocytes, macrophages and - to some extent - DCs is dependent on the cytokine and hematopoietic growth factor receptor Csf1r (c-fms, M-CSFR, CD115), expressed in monocytes, macrophages, and mononuclear phagocyte precursors (14-17). Characterization of op/opmice, a spontaneous mutant lacking a functional Csf1gene, has revealed both the role of Csf1 in the development of mononuclear phagocytes, and also their broad functions. (17). The known ligands of Csf1r, Csf1/M-CSF (18)and interleukin (IL)-34 (19), are likely both important for the development of the mononuclear phagocyte lineage, as M-CSF-deficient mice have a milder phenotype than Csf1r-deficient mice. Another cytokine receptor closely related to Csf1r, fms-related tyrosine kinase 3 (FLT3 also known as Flk2) receptor, is critical for the development of cDCs and PDCs (10, 20-22).
Is MDP differentiated into DCs?
Differentiation of MDP into monocytes and macrophages is less well understood than their differentiation into DCs. Among differentiated ‘mature’ monocytes in the bone marrow, blood and spleen, at least two phenotypic and functional subsets can be identified (reviewed in (3)), whose developmental relationship is still unclear. Adoptive transfer experiments demonstrate that bone marrow Gr1+/Ly-6Chighmonocytes can shuttle between the blood and the bone marrow, and lose Ly-6C expression (25, 42), suggesting that they give rise to Gr1-/Ly-6Clowmonocytes. However, neither a genetic defect in or antibody-mediated depletion of Gr1+/Ly-6Chighmonocytes affect the generation of Gr1-/Ly-6Clowmonocytes (43-46). When bone marrow is used a source of monocytes, current adoptive transfer protocols may not always distinguish mature Gr1+/Ly-6Chighmonocytes from their proliferating precursors because pre-monocytes are ill-defined. Therefore, a better characterization of monocyte precursors and new lineage tracking studies will be needed to establish the developmental relationship between monocyte subsets.
Do monocytes give rise to cDCs?
1); however, it is now widely accepted that monocytes do not give rise to cDCs and PDCs (9, 24).
Where are plasmacytoid DCs found?
They are present in the bone marrow and all peripheral organs . PDCs are specialized to respond to viral infection with a massive production of type I interferons (IFN), however, they also can act as antigen presenting cells and control T cell responses(13).
How do monocytes proliferate?
Classical monocytes are well-characterized. In response to infection or injury, they proliferate in the bone marrow, are released into circulation in a CCR2-dependent manner and home to the site of interest via a chemokine gradient (3). During bacterial infection, for example, these monocytes home to the site of infection and phagocytose pathogens, secrete a distinct set of chemokines that lead to recruitment of other immune cells, and present antigen via class II MHC (4). Murine studies have also revealed that these monocytes can exit the vasculature, and without further differentiating, survey the tissue microenvironment, before departing via the lymphatics (5). The spleen functions as a reservoir for monocytes. In response to signals emanating from a distant tissue injury, for instance release of angiotensin II during myocardial infarction, these cells can be mobilized to the site of injury from the spleen (6).
What are the roles of monocytes and macrophages in the immune system?
Both monocytes and macrophages utilize pattern recognition-receptors to recognize pathogens and initiate their response . Classical monocytes are mobilized in response to bacterial, fungal, protozoal, and viral pathogens (35). On arrival to inflamed or infected tissue, they differentiate into either dendritic cells or macrophages. This distinction is not clear-cut, but rather based on cytokine profile and immunophenotyping. For example, in models of mouse colitis, Ly6Chighmonocytes are recruited to the intestine and give rise to CX3CR1+dendritic cells which then phagocytose bacteria and transport them to mesenteric lymph nodes to induce T-cell response (36). The same circulating Ly6Chighmonocytes are capable of differentiating into TNF-α and inducible nitric oxide producing DCs or differentiate into macrophages which, depending on environmental cues, can either contribute to a pro-inflammatory milieu or resolution of inflammation (37, 38). Current guidelines recommend referring to these cells as simply “monocyte-derived cells” or MoDCs to avoid confusion, as the functions can overlap (39).
What cells differentiate into pulmonary dendritic cells?
Utilizing a combination of fate-mapping techniques, parabionts, and fluorescent reporter murine studies, investigators have shown that Ly6Chighclassical monocytes differentiated into both the CD11b+CD103−and CD11b−CD103+dendritic cell subsets (7). In addition, these cells differentiate into macrophages when recruited to the lung and help replenish the tissue resident macrophage pool when depleted either by injury or through experimental manipulation (8).
What are the roles of monocytes in lung inflammation?
Monocytes and macrophages are emerging as key players in mediating both the pathogen response and sterile lung inflammation, including that arising from barotrauma and ischemia-reperfusion injury. Ongoing studies will establish the mechanisms by which these monocytes and macrophages initiate a variety of immune response that lay the fundamental basis of injury response in the lung.
What is the role of macrophages in inflammatory response?
In response to extra- or intra-cellular pathogens, M1 macrophages upregulate inducible nitric oxide synthase and secrete pro-inflammatory chemokines and cytokines via pattern recognition receptors. They also present antigen via MHC class II, initiating inflammation, recruitment of granulocytes, and a Type-1 helper T cell response. The second, “alternative” or “M2” activation is more varied in its phenotype. In response to IL-4 and IL-13 during allergy response or parasite infection, M2 macrophages secrete histamine and promote killing and encapsulation of parasites. Outside of the realm of pathogen response, M2-activated macrophages are capable of down-regulating the initial inflammatory response and promoting the resolution of inflammation and initiation of tissue healing and fibrosis (27). Importantly, the M1/M2 classification of activation is likely too dichotomous in its characterization and the actual activation states of macrophages likely is better represented along a continuum in response to a variety of stimuli with responses ranging from pro-inflammatory to anti-inflammatory, demonstrating the remarkable plasticity of macrophages (28, 29).
Which cells are critical for host innate immune defense against pathogens?
Monocytes and macrophages in the lung are crucial players in host innate immune defense against pathogens.
What are non-classical monocytes?
They display a distinct motility and crawling pattern . In vivoimaging studies have shown non-classical monocytes crawling along the luminal side of the endothelium. This integrin-dependent crawling is independent of the direction of blood flow. In fact, it is frequently against the direction of flow (11, 12). In the lung, non-classical monocytes are known to be capable of differentiating into CD11b+CD103−dendritic cells (7). Recent studies have further attempted to explore the role of these monocytes. Investigators have shown that non-classical monocytes are involved in intraluminal surveillance of the endothelium and phagocytosis of injured endothelium along with recruitment of neutrophils to the site of injury (13). In addition, these cells have also been shown to limit tumor metastases to the lung via the CX3CR1-CX3CL1 axis (14). Studies of human non-classical monocytes have shown that these monocytes sense nucleic acids and viruses via TLR7 signaling and can initiate the innate immune response by secreting cytokines (15). These monocytes can be mobilized from the marginalized vascular compartment during vigorous exercise, sepsis, and after cardiac surgery (16–18) and are depleted by high-dose glucocorticoid treatment (19, 20).
What is the Difference Between Monocyte and Macrophage?
Monocytes and macrophages are two types of white blood cells. Monocytes are the largest type of white blood cells that have the ability to differentiate into macrophages and dendritic cells. On the other hand, macrophages are specialized cells that involve in innate immunity by engulfing infectious particles. This is the key difference between monocyte and macrophage. Another difference between monocyte and macrophage is their size; a monocyte is larger than a macrophage. Furthermore, monocytes are present in the bloodstream, whereas macrophages are present in the extracellular fluid that bathes tissues. Therefore, this is also a difference between monocyte and macrophage.
What happens when monocytes reach an organ?
Once monocytes reach an organ or a tissue from the bloodstream, they will differentiate into macrophages. Macrophages are large, irregular shaped, agranulated cells with a large bean-shaped nucleus. They are capable of engulfing foreign particles, which could be a threat to human health or cause diseases to humans. We call this engulfing process phagocytosis. Once they engulf foreign particles, they form a membrane-bounded phagosome surrounding them. Then the lysosomes release their enzymes in order to kill, and digest engulfed particles. In addition, rapidly produced oxygen-containing free radicals in phagosomes also help to degrade the pathogens.
What is a Monocyte?
Monocytes are irregular shaped white blood cells that circulate in the bloodstream. Unlike other white blood cells, monocytes are large and have a bean-shaped nucleus in the cell. When monocytes enter an organ or tissue from the bloodstream, they differentiate into cells called ‘macrophages’; thus monocytes are the precursor cells of macrophages.
What is the function of a macrophage?
Figure 02: Macrophage. Macrophages are capable of engulfing bacteria, viruses, cellular debris, and dust particles in the lungs. When an infection occurs in a tissue or an organ, monocytes in the bloodstream squeeze through the epithelium cells and enter the site of infection. At the site of infection, the monocytes differentiate into active, ...
Why are agranulocytes and phagocytosis the same?
Both these cell types are agranulocytes due to the absence of cytoplasmic granules. These two types of cells have similar roles in the immune system such as phagocytosis, presenting antigens to T lymphocytes, and production of cytokine that help to initiate and coordinate immune responses.
What percentage of white blood cells are monocytes?
About 3 – 8% of the white blood cells are monocytes in the human circulatory system. All white blood cells are derived from progenitor cells. However, in this case, progenitor cells are differentiated into monoblast and then into promonocytes. Promonocytes are finally differentiated into monocytes.
What are the different types of cells in the immune system?
The immune system has different types of cells including lymphocytes, macrophages, monocytes, neutrophils, and other cells such as basophils, eosinophils, and natural killer cells . Macrophages and monocytes are the large white blood cells with an irregular shape; they stimulate antibody production in the body.