How do polyatomic ions work in ionic compounds? A polyatomic ion has two or more covalently bonded atoms that act as a single ion. The polyatomic ion forms ionic bonds with other ions and acts externally as a unit, just like monatomic ions.
How are polyatomic ions formed?
Polyatomic ions are created when a number of atoms come to together to form a group, and then that group has either a positive or a negative charge, which means that it can be either a cation or an anion. We discuss how protons and electrons balance to give a net charge, and then discuss some common polyatomic ions.
What is the electrical charge of a polyatomic ion?
Many of the polyatomic ions have an electrical charge of -1. Polyatomic ions with a -2 charge are also common. However, other polyatomic ions form with the -3 charge, but the borate and phosphate ions are the ones to memorize. Q. How many protons and electrons are in a hydroxide ion?
Why do polyatomic ions have more electrons than neutral atoms?
Therefore compared to the neutral atom, they have extra electrons for negatively charged anion or not enough electrons for the positively charged cation. Many common chemicals are polyatomic compounds with polyatomic ions. For example, sulfuric acid H 2 SO 4 contains H + and the polyatomic SO 4-2.
What is an example of a polyatomic compound?
Many common chemicals are polyatomic compounds with polyatomic ions. For example, sulfuric acid H 2 SO 4 contains H + and the polyatomic SO 4-2. The sulfur atom has six electrons in its outer shell. It shares them covalently with the oxygen atoms having six electrons in their outer shells.

How polyatomic ions get their charge?
Where does the charge come from? Polyatomic ions get their charge by transferring electrons from another element. For example In NaNO3 the Na loses an electron and the NO3 gains the electron.
What causes polyatomic ions to form?
0:175:14What's a polyatomic ion? - YouTubeYouTubeStart of suggested clipEnd of suggested clipMinus polyatomic ions or what happens when more than one atom. Comes together and they form a bigMoreMinus polyatomic ions or what happens when more than one atom. Comes together and they form a big group of atoms. And then that group of atoms. Itself has a charge. So polyatomic ions are things like
What are the rules for polyatomic ions?
Rule 1. The cation is written first in the name; the anion (takes electrons) is written second in the name. Rule 2. When the formula unit contains two or more of the same polyatomic ion, that ion is written in parentheses with the subscript written outside the parentheses.
How does polyatomic ions bond?
Recall that a polyatomic ion is a group of atoms that are covalently bonded together, and which carry an overall electrical charge. The ammonium ion, NH+4, is formed when a hydrogen ion (H+) attaches to the lone pair of an ammonia (NH3) molecule in a coordinate covalent bond.
What is the easiest way to learn polyatomic ions?
2:189:54Tricks for Remembering Polyatomic Ions - YouTubeYouTubeStart of suggested clipEnd of suggested clipIf you remember us looking at that list just a minute ago all of our polyatomic ions had a firstMoreIf you remember us looking at that list just a minute ago all of our polyatomic ions had a first symbol to it and then an oxygen with it and then a certain number of oxygens with the charge.
What happens to a polyatomic ion when it dissolves in water?
Polyatomic ions have a central atom which is covalently bonded to its surrounding atoms forming a unit, which has a charge as a whole. They do not dissociate in water due to the covalent bonding. Hence, polyatomic ions remain intact in water. Whereas, acids dissociate in water to form ions.
How do you determine if an ion is polyatomic?
All the elements on the periodic table start with a capital letter and only some of them have a second letter that is lower case. So if you see two capital letters together in a ion then you will know that it is a polyatomic.
Which statement is true about a polyatomic ion?
Which statement is true of a polyatomic ion? It contains one ion with two or more atoms.
How do you tell if a compound contains a polyatomic ion?
Polyatomic ions are ions which consist of more than one atom. For example, nitrate ion, NO3-, contains one nitrogen atom and three oxygen atoms. The atoms in a polyatomic ion are usually covalently bonded to one another, and therefore stay together as a single, charged unit. Rule 1.
What type of bonds do polyatomic ions form?
Polyatomic ions are ions that are composed of two or more atoms that are linked by covalent bonds, but that still have a net deficiency or surplus of electrons, resulting in an overall charge on the group. A metal plus a polyatomic ion yields an ionic compound.
Where do the extra electrons come from in polyatomic ions?
Re: Where/how do polyatomic ions obtain extra electrons? The electrons to make anions must come from electron donor atoms/molecules. Those are called cations and include elements such as sodium, potassium, calcium and many other elements on the left side of the periodic table or many other molecules.
Why do polyatomic ions have covalent bonds?
Because the compound contains an extra electron, the overall charge is minus one. In conclusion, the various elements are held together with covalent bonds, but the compound possesses an overall charge, so that the entire compound behaves as ion and can be used in ionic bonding.
Do polyatomic ions form ionic bonds?
Polyatomic ions are ions that are composed of two or more atoms that are linked by covalent bonds, but that still have a net deficiency or surplus of electrons, resulting in an overall charge on the group. A metal plus a polyatomic ion yields an ionic compound.
Which statement is true about a polyatomic ion?
Which statement is true of a polyatomic ion? It contains one ion with two or more atoms.
Which polyatomic ions will form bonds with negatively charged ions?
Polyatomic Ion NH4+ or Ammonium Most polyatomic ions contain oxygen and are negatively charged anions because the oxygen atoms attract electrons.
Where do the extra electrons come from in polyatomic ions?
Re: Where/how do polyatomic ions obtain extra electrons? The electrons to make anions must come from electron donor atoms/molecules. Those are called cations and include elements such as sodium, potassium, calcium and many other elements on the left side of the periodic table or many other molecules.
What are 3 examples of polyatomic ions?
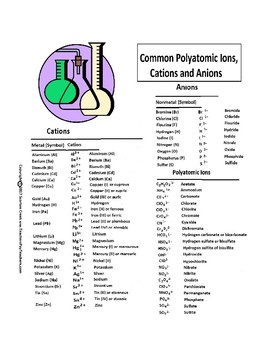
Examples of polyatomic ions are Acetate (CH 3 COO – ), Phosphate (PO 4 3- ), Permanganate (MnO 4 – ) , Oxalate (C 2 O 4 2- ) etc.
What is a polyatomic element?
The is made up of two or more atoms, it can be referred to as a polyatomic ion or a molecular ion. Depending on the charge it may be classified a...
Is phosphate a polyatomic ion?
Phosphate (PO 4 3- ) is a polyatomic ion carrying a negative charge. The phosphate ion made more than one atom.
What are monatomic ions called?
The ion is made up of only one type of atom each holding some net charge, positive or negative, it can be referred to as a monatomic ion.
Is Mg 2+ a polyatomic ion?
No Mg 2+ ion is a monatomic ion, it is made up of only one Mg atom.
How to find the total number of protons in a hydroxide ion?
Ans: We can calculate the total number of protons in a hydroxide ion by adding up the number of protons in one hydrogen atom and one oxygen atom:
What is a polyatomic ion?
Polyatomic ions are covalently bonded groups of atoms and having a positive or negative charge caused by the formation of an ionic bond with another ion. Compounds formed from such a combination of ions are polyatomic ionic compounds.
Why do monatomic ions have a net charge?
The ion has a net charge over it because the total number of electrons is not balanced by the total number of protons in the nucleus.
Why do polyatomic ions behave differently?
This is because there are two or more atoms present in the polyatomic ion.
What are some examples of monatomic ions?
Thus it is different from monatomic ions, which contain only one atom. Examples of monatomic ions include Na+ and Cl- etc. This article will give details of polyatomic ions and their examples.
What type of ions have a -2 charge?
Polyatomic ions with a -2 charge are also common.
How many electrons does ammonium have?
When ammonium forms an ionic bond with an OH the extra electron transfers to complete the outermost shell of the OH. It needs two electrons but has only one from the OH hydrogen atom. The electron from the NH 4 transfers to the OH creating an OH – ion and an NH 4+ ion.
Why alphabetize polyatomic ions?
I would suggest alphabetizing the polyatomic ions so you can reuse your associations by following them along a journey.
What is the NH4+ ion part?
Here’s what I mean in context of ammonium carbonate: The NH4+ ion part is the positively charged polyatomic atom we want to memorize. Nick is a great association for ‘N’.
Why did I choose Nick?
The reason I chose Nick is because his name starts with ‘N’. This reminds me that the first polyatomic atoms I’m going to memorize in this Memory Palace also start with ‘N.’ The rest are in alphabetical order to also reduce unnecessary thinking. ‘P’ is the next letter after ‘N’ for the purposes of this list.
Why is alphabetization important in the Magnetic Memory Method?
I’ve organized them this way because alphabetization is a key principle of the Magnetic Memory Method. It helps reduce cognitive overload. Organizing information in this way also helps maximize the core aspects of memory that make the Memory Palace technique work so well:
What is the perfect association for the number 2?
Swans are a perfect association for the number 2. Adding a sword can help memorize that the ion is negative.
What does the 4 symbol look like?
Heath Ledger is a great celebrity to use as an association for ‘H.’. ‘4’ looks a lot like a sailboat, and the + symbol looks like a cross. “Ion” doesn’t really require an image because I assume we all know that it belongs to the core information.
What to do if memory techniques don't suit you?
If for any reason, the memory techniques you’ve learned today don’t suit you, try mind mapping instead.
Why do I need to memorize polyatomic ions?
Polyatomic ions are important in chemistry because they are found in many common chemical compounds. Because of this, it is helpful to memorize the most common of these ions. Some teachers do allow students to use a list of polyatomic ions for exams while others do not.
What is the easiest way to learn chemical formulas?
Use the Periodic Table. To write chemical formulas, acquaint yourself with chemical symbols, most easily found on the periodic table of elements.
How do you name polyatomic ions for dummies?
Ionic compounds involving polyatomic ions follow the same basic rule: Write the name of the metal first, and then simply add the name of the nonmetal (with the polyato mic anions, it is not necessary to add the -ide ending).
What is the hint for working with polyatomic ions?
In a similar fashion to the suffix pattern, the prefix pattern involved in naming polyatomic ions shows extreme values of oxygen atoms in the ions. The two important prefixes are “ per” and “hypo.” If an ion has a “per” prefix, it means the ion has one more oxygen atom than does the ion with as “ate” suffix.
How do you know how many oxygens are in a polyatomic ion?
Patterns for Polyatomic Oxyanions Notice how the four polyatomic-ate ions in the center square (phosphate, arsenate, sulfate, and selenate) all have four oxygen atoms, while the polyatomic -ate ions on the outside all have three oxygen atoms.