You balance an equation with parentheses the same way as with other equations. You must remember to multiply the subscripts of atoms inside the parentheses by the subscripts outside the parentheses. For example, balance the equation:
What are some basic rules for balancing chemical equations?
To balance equations on your own, follow these simple rules:
- Check that all the formulae in the equation are correct.
- Deal with only one element at a time.
- Balancing is adding BIG numbers. You cannot change any of the small numbers in a chemical formula. If balancing is required, put the number in front of the substance.
- Check each element again and repeat step 3 again if needed.
What are the steps for balancing chemical equations?
Steps to Balancing Chemical Equations 1. Count the number of atoms of each element in the reactants and the products. List each element and how many atoms it has underneath the reactants and products. Example: HCl + Na 2 S H 2 S + NaCl H – 1 H – 2 Cl – 1 Cl – 1 ...
How do you write a balanced chemical equation?
Write Down Number of Atoms. The next step for balancing the chemical equation is to determine how many atoms of each element are present on each side of the arrow: Fe + O 2 → Fe 2 O 3. To do this, keep in mind a subscript indicates the number of atoms. For example, O 2 has 2 atoms of oxygen.
What is the best way to balance chemical equations?
Steps
- Take the unbalanced equation and make a note of the elements present in each side of the equation.
- Now, count the number of molecules of each element present on both sides of the equation.
- Here comes the task of balancing the chemical equations. ...
What does parentheses mean in a compound?
What is the purpose of chemical equations?
What is the equation for the reaction of aluminium and hydrochloric acid?
How to get rid of denominators?
What are some examples of reactions between water and calcium?
About this website
3_Figure2.png)
How to balance a chemical equation?
Follow four easy steps to balance a chemical equation: 1 Write the unbalanced equation to show the reactants and products. 2 Write down how many atoms of each element there are on each side of the reaction arrow. 3 Add coefficients (the numbers in front of the formulas) so the number of atoms of each element is the same on both sides of the equation. It's easiest to balance the hydrogen and oxygen atoms last. 4 Indicate the state of matter of the reactants and products and check your work.
How do you know if an equation isn't already balanced?
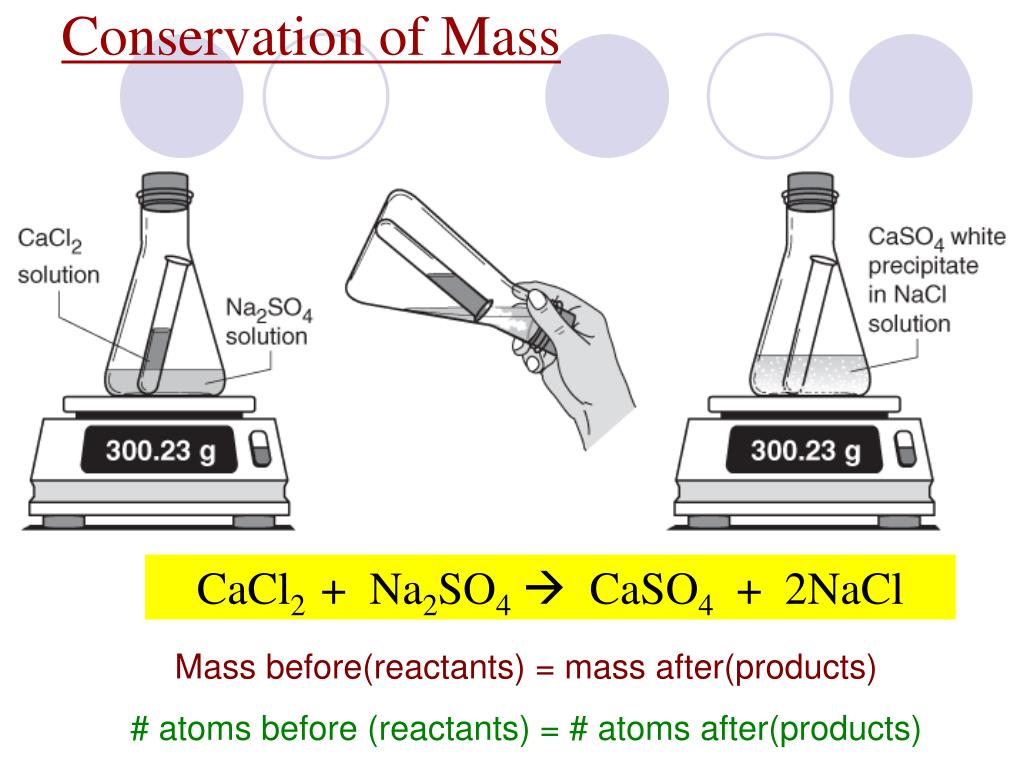
How do you know the equation isn't already balanced? Because the number of atoms on each side isn't the same! Conservation of Mass states mass isn't created or destroyed in a chemical reaction, so you need to add coefficients in front of the chemical formulas to adjust the number of atoms so they will be the same on both sides.
What is coefficient in math?
You add coefficients . Coefficients are whole number multipliers. If, for example, you write 2 H 2 O, that means you have 2 times the number of atoms in each water molecule, which would be 4 hydrogen atoms and 2 oxygen atoms.
What is the starting material of a reaction?
The starting materials, called reactants , are listed on the lefthand side of the equation. Next comes an arrow that indicates the direction of the reaction. The righthand side of the reaction lists the substances that are made, called products .
Why doesn't 3 Fe work on the left?
3 Fe doesn't work on the left because you can't put a coefficient in from of Fe 2 O 3 that would balance it.
Where do reactants go in chemistry?
Note the reactants always go on the left side of the arrow. A "plus" sign separates them. Next, there is an arrow indicating the direction of the reaction (reactants become products). The products are always on the right side of the arrow. The order in which you write the reactants and products is not important.
Can you write a balanced equation using multiples of the coefficients?
Note: You could have written a balanced equation using multiples of the coefficients. For example, if you double all of the coefficients, you still have a balanced equation:
What does parentheses mean in a compound?
The parentheses mean there are 2 or more of what are contained in the parenthses in the compound. Since balancing means getting the same total numbers of each type of atom on each side, you must calculate the total number of atoms in the formula.
What is the purpose of chemical equations?
Chemical equations are used to describe reactions between two or more substances. To identify of a chemical reaction, we first have to identify what reactants are involved and what product is produced.
What is the equation for the reaction of aluminium and hydrochloric acid?
The word equation for this reaction is aluminium + hydrochloric acid -> Aluminium chloride + hydrogen
How to get rid of denominators?
To get rid of the denominators, simply multiply each number by the LCM of the denominators. Multiplying each coefficient by 6, we get:
What are some examples of reactions between water and calcium?
Another example: reaction between calcium and water. It produces calcium hydroxide and hydrogen.
