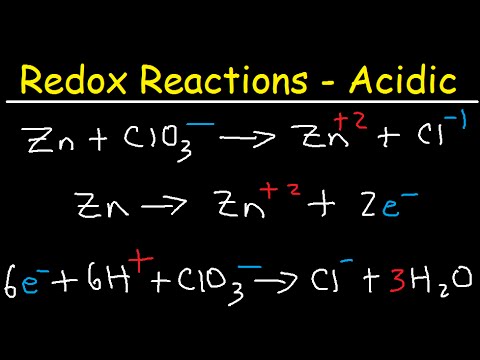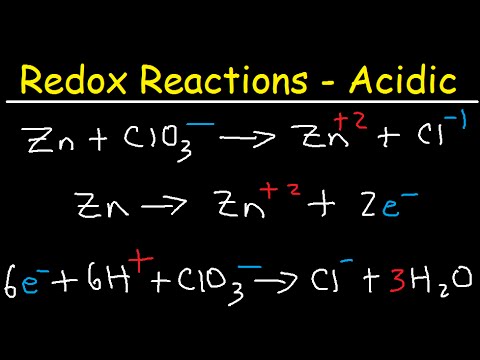
To write a balanced oxidation or reduction reaction for a species in acidic aqueous solution:
- Write a skeletal equation for the oxidation or reduction equation based on the information provided.
- Balance the half-reaction equation according to the following sequence: (i) Balance all atoms other than H and O by inspection (ii) Balance O atoms by adding H 2 O to the appropriate side ...
- Balance the charge by adding electrons (e -) to the side deficient in negative charge.
- Step 1: Divide the reaction into half reactions.
- Step 2: Balance the elements other than H and O. ...
- Step 3: Balance the O atoms by adding H2O.
- Step 4: Balance the H atoms by adding H+
- Step 5: Balance the charges by adding e-
- Step 6: Add the half reactions and simplify.
How do you balance half reactions step by step?
In summary: Step 1: Break reaction into half-reactions by ions. Step 2: Balance the half-reactions stoichiometrically by adding water, hydrogen ions (H +) and hydroxyl ions (OH -) to the half-reactions. Step 3: Balance the half-reactions charges by adding electrons to the half-reactions.
Which half-reaction should be balanced in an acid solution?
Here is the half-reaction to be considered: MnO4¯ ---> Mn2+ It is to be balanced in acid solution. Here is a second half-reaction: Cr2O72¯ ---> Cr3+[acid soln] As I go through the steps below using the first half-reaction, try and balance the second half-reaction as you go from step to step. The answer will appear at the end of the file.
How do you balance the particles in a chemical reaction?
First, separate the reaction into two half reactions. Balance the number of atoms and the charge on both sides. In acidic solution, you can add H+ and H2O to balance the particles. In Basic solution, you can use OH- and H2O to do so.
How do you balance the equation for a redox reaction in acid?
When balancing equations for redox reactions occurring in acidic solution, it is often necessary to add H⁺ ions or the H⁺/H₂O pair to fully balance the equation. In this video, we'll walk through this process for the reaction between dichromate (Cr₂O₇²⁻) and chloride (Cl⁻) ions in acidic solution.. Created by Jay.

How do you balance half-reactions in basic solutions?
Step 1: Write the unbalanced ionic equation. Step 2: Write separate half-reactions for the oxidation and the reduction processes. Step 3: Balance the atoms in the half-reactions other than the hydrogen and oxygen. Step 4: Balance oxygen atoms by adding water molecules to the appropriate side of the equation.
How do you balance a redox reaction by oxidation number method in acidic medium?
0:348:45Balancing Redox Reactions by Oxidation Number Method - YouTubeYouTubeStart of suggested clipEnd of suggested clipEqual place these numbers as coefficients before the formulas containing those atoms. If theMoreEqual place these numbers as coefficients before the formulas containing those atoms. If the reaction is taking place in water add h plus or o h minus on appropriate side for the charge. Balance.
How do you know if a redox reaction is acidic or basic?
If H+ or any acid appears on either side of the chemical equation the reaction takes place in the acidic solution. If OH- or any base appears on either side of the chemical equation the solution is basic.
How do you write a half-reaction of a redox reaction?
Steps to Writing Half-Reactions of Redox ReactionsStep 1: Write the unbalanced redox reaction in its ionic form. ... Step 2: Write the oxidation state of each species in the reaction. ... Step 3: Write the two half-reactions. ... Step 4: Determine which reaction is the oxidation reaction and which is the reduction reaction.More items...
What is the difference between acidic and basic solutions in working with redox reactions?
So the only difference between reactions in basic and acidic conditions is that blaancing reactions in a base, you would first balance it like how you would in an acid, but since you're in a basic solution now, you would add enough OH- to both sides of the equation to neutralize the H+.
How do you balance acid base reactions?
0:227:26Balancing Acid Base Reactions: Stoichiometry Part 3 by Leah4sciYouTubeStart of suggested clipEnd of suggested clipYou treat it just like any other reaction where you have to have the same number of atoms in yourMoreYou treat it just like any other reaction where you have to have the same number of atoms in your reactants match those atoms in the products. The first thing to do is complete this reaction.
What is the difference between acidic medium and basic medium?
Differences Between Acid and Base Acid is a kind of chemical compound that when dissolved in water gives a solution with H+ ion activity more than purified water. A base is an aqueous substance that donates electrons, accepts protons or releases hydroxide (OH-) ions. An acid is a proton donor.
How do you know if an equation is acidic or basic?
A solution's pH will be a number between 0 and 14. A solution with a pH of 7 is classified as neutral. If the pH is lower than 7, the solution is acidic. When pH is higher than 7, the solution is basic.
How do you balance redox reactions with oxidation numbers?
The only sure-fire way to balance a redox equation is to recognize the oxidation part and the reduction part. Then you balance by making the electron loss equal the electron gain. In the oxidation number method, you determine the oxidation numbers of all atoms.
How do you balance redox equations using oxidation numbers?
5:118:28Redox Balancing | Oxidation Number Method - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe need 6 electrons multiplied by 2 and it will produce its electrons. So the number of electronsMoreWe need 6 electrons multiplied by 2 and it will produce its electrons. So the number of electrons lost in the oxidation half-reaction is equal to the number of electrons gained.
How do you balance redox reactions with oxidation states?
1:266:04Chemistry 13.5 Balancing Redox by Oxidation Number - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo for copper the copper starts as a zero this one's zero and it ends up as a two plus right hereMoreSo for copper the copper starts as a zero this one's zero and it ends up as a two plus right here the silver on the other hand. Starts as a plus one we can see that right here and ends up as a zero.
What is the basis of balancing of redox reaction by oxidation number method?
Oxidation number method is based on the difference in oxidation number of oxidizing agent and the reducing agent. Half-reaction method depends on the division of the redox reactions into oxidation half and reduction half.
Step 2: Balance the elements other than H and O
Fortunately, all atoms other than H and O are already balanced, so we can move on to the next step.
Step 6: Add the half reactions and simplify
Because the number of electrons in the first half reaction (3e-) does not equal the number of electrons in the second half reaction (2e-), we must multiply the reactions by coefficients so that the electrons will cancel out when we add them.
Step 2: Balance the elements other than H and O
Fortunately, all atoms other than H and O are already balanced, so we can move on to the next step.
Step 9: Add the half reactions and simplify
Recall that we must multiply the reactions by coefficients so that the electrons will cancel out when we add them. In this case, we multiply the first reaction by 2 to yield 6e-, and the second reaction by 3 to yield 6e- as well.
How to balance half reaction?
Here are the 4 acid steps: 1) Balance the atom being reduced/oxidized. 2) Balance the oxygens (using H 2 O). 3) Balance the hydrogens (using H + ). 4) Balance the charge.
What does "balanced as if in acid solution" mean?
1) Balanced as if in acid solution; there were no oxygens to balance.
What is the term after a half reaction?
4) A term like "base" or "basic soln" is written after the half-reaction. Usually in parentheses or square brackets.
Why is water present in a redox reaction?
The water is present because the reaction is taking place in solution, the hydroxide ion is available because it is in basic solution and electrons are available because that's what is transfered in redox reactions. Remember, these three are always available, even if not shown in the unbalanced half-reaction presented to you in the problem.
Where does the answer appear in the balancing technique?
The answer will appear at the end of the file. Before looking at the balancing technique, the fact that it is in basic solution can be signaled to you in several different ways: 1) It is explicitly said in the problem. 2) A base (usually a strong base) is included as one of the reactants.
Which two elements were already balanced?
The Ni and O were already balanced. Only H+and e¯ were needed.
Do redox half reactions have to be balanced?
Reminder: a redox half-reaction MUST be balanced both for atoms and charge in order to be correct. It is VERY easy to balance for atoms only, forgetting to check the charge.
How to balance oxygen on both sides?
Balance the oxygen on both sides by adding H 2 O to one side.
How to get a full redox reaction?
To get a full redox reaction, we need to add together a reduction half-reaction and an oxidation half-reaction. However, before doing so we need to multiply both half-reactions by coefficients so that all freestanding electrons will cancel. This is the key to getting full, balanced redox reactions.
What ions combine to make water?
Combine all hydrogen and hydroxide ions on the same side into water.
Is hydrogen balanced on the left side?
Now the hydrogen is not balanced. There are 12 more hydrogen on the left side, so add that many ions to the right side:
Which element is balanced with oxygen?
The aluminum and rhenium are balanced, so we start with the oxygen:
Do half reactions need to be balanced?
Oftentimes the half-reactions will not be balanced beforehand, so we need to balance them. Examples 8 and 9 let you work through the whole process.
Can we combine half reactions?
We can combine the half-reactions, but first equate the electrons; multiply the potassium half-reaction by 4:
How to balance charge after all atoms are balanced?
After all the atoms have been balanced we will balance the charge by adding electrons to the side with the greatest positive charge (deficient in electrons).
Which side of the equation does H+ add to?
There are no H atoms on the right hand side of the equation, so we add H+ to the right hand side:
What is the oxidation of alkanols?
oxidation of primary alkanols produces alkanals then alkanoic acids. We can write balanced half-equations for the oxidation of an aqueous solution of alkanol, for example ethanol, to an alkanoic acid, for example acetic acid (ethanoic acid), under acidic conditions.
What happens when an oxidation reaction involves a species losing electrons?
Recall that an oxidation reaction involves a species losing electrons, that is, electrons will be a product in the half-equation.
What is the species that will be reduced in a reduction reaction?
Remember that for a reduction reaction electrons should be a reactant in the equation. Oxidising agents, or oxidants, are the species that will be reduced. Oxidising agents (oxidants) you may be familiar with are the dichromate ion (Cr 2 O 72-(aq)) and the permanganate ion (MnO 4-(aq) ).
How to balance a negative charge?
Balance the charge by adding electrons (e -) to the side deficient in negative charge.
Which side of the atom is 4 O?
4 O atoms are present on the left hand side.
What happens when an atom loses hydrogen?
Look at the basic laws of the chemical reaction is it losing or gaining a hydrogen, if an atom is losing a hydrogen it is going to be an acid in which it becomes a conjugate base and if it is gaining a hydrogen atom it is a base in which it becomes a conjugate acid. 3 comments.
Where does the H+ ion come from?
The H+ ions come from the acidic solution as well, as they are present in all acidic solutions.
How many oxidation numbers does Cr have?
So the 2 chromium atoms need to have oxidation numbers that equal +12 and there are 2 of them so each Cr has an oxidation number of +6.
How many oxidation states does oxygen have?
I do believe, however, that each individual oxygen atom does have a 2- oxidation state, which would total 14-, but then each chromium atom has a 6+ oxidation state, cancelling out 12 of those negative charges (because there are two chromium atoms), thus resulting in the 2- overall charge of the molecule.
Is Cr2O7 a single unit?
Cr2O7 (dichromate) is a polyatomic ion. It was bonded to something else but dissolved in a solution. It is easier to think of polyatomics as single units. Since it is already 1 ion, it cannot ionize, or split, anymore. Comment on Steven "Trey" Kline's post “Cr2O7 (dichromate) is a polyatomic ion. It was bon...”.
How to balance half reactions?
In summary: Step 1: Break reaction into half-reactions by ions. Step 2: Balance the half-reactions stoichiometrically by adding water, hydrogen ions (H +) and hydroxyl ions (OH -) to the half-reactions. Step 3: Balance the half-reactions charges by adding electrons to the half-reactions. Step 4: Multiply each half-reaction by a constant so both ...
What is the half reaction method?
This is called the half-reaction method of balancing redox reactions , or the ion-electron method. Each half-reaction is balanced separately and then the equations are added together to give a balanced overall reaction.
How to balance iodine atoms?
To balance the atoms of each half-reaction, first balance all of the atoms except H and O. For an acidic solution, next add H. Balance the iodine atoms: 2 I - → I 2. The Mn in the permanganate reaction is already balanced, so let's balance the oxygen: MnO 4 - → Mn 2+ + 4 H 2 O. Add H + to balance the water molecules: