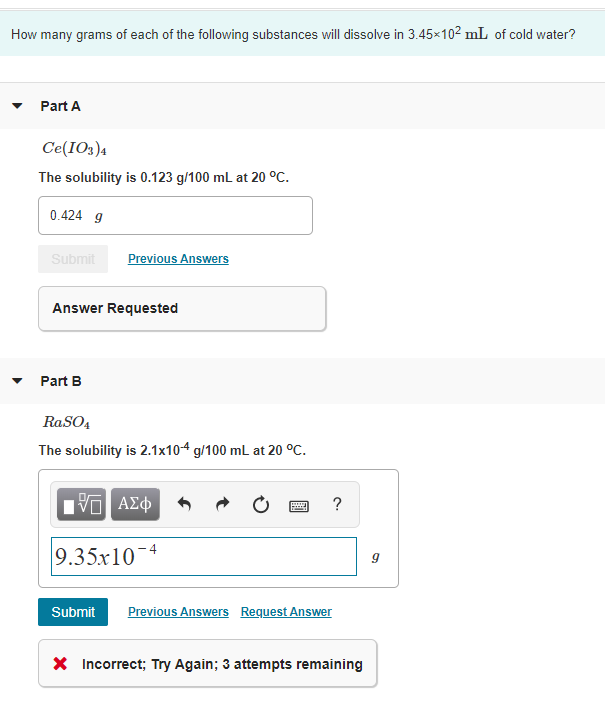
Here is how to convert a g/100mL value to molar solubility: 1) multiply the g/100mL value by 10/10. This converts it to grams per 1000 mL or, better yet, grams per liter. (Sometimes the data is given in g/L. When that happens, this step is skipped.) 2) divide the grams per liter value by the molar mass of the substance.
How do you calculate grams per 100 ml solubility?
Calculating gram per 100 mL solubility, given the Ksp. Return to Equilibrium Menu. Basically, this type of problem will (1) calculate the molar solubility from the Ksp. Then, in an additional step, (2) calculate grams per liter from moles per liter. From there, it is an easy third step to g/100mL.
What is solubility and how is It measured?
Since the solubility is temperature-dependent there should be uniform temperature throughout the system when dissolving substances. Solubility is measured either in grams per 100 g of solvent g/100g or number of moles per 1 L of the solution Formula to calculate solubility.
How do you calculate KSP from molar solubility?
2) divide the grams per liter value by the molar mass of the substance. This gives moles per liter, which is molar solubility. After the above conversion, the problem becomes calculate the Kspfrom molar solubility data.
How much solubility is required for a single solute?
Very slightly soluble. If the chemical requires 1,000 to 10,000 parts of solvent for a single part of solute. Slightly soluble. If the chemical requires 100 to 1,000 parts of solvent for a single part of solute.

How do you find grams from KSP?
0:417:16WCLN - Mass from Ksp - Chemistry - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe molar solubility of cui l3 - is just equal to its concentration. In a saturated solution. SoMoreThe molar solubility of cui l3 - is just equal to its concentration. In a saturated solution. So once we have this concentration. We convert it to moles. And then to mass and grams.
How do you find how many grams dissolved in water?
4:017:58Calculating how much Solute will Dissolve - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo doing that for everyone to get the 100 grams is right here for every 100 grams of water. We wouldMoreSo doing that for everyone to get the 100 grams is right here for every 100 grams of water. We would say approximately right we're at 15 approximately 8 grams of kclo3.
How do you calculate solubility product?
In this case, we calculate the solubility product by taking the solid's solubility expressed in units of moles per liter (mol/L), known as its molar solubility. The concentration of Ca2+ in a saturated solution of CaF2 is 2.1 × 10–4 M; therefore, that of F– is 4.2 × 10–4 M, that is, twice the concentration of Ca2+.
How do you measure the solubility?
A widely accepted and accurate method for measuring the solubility is through equilibration of a suspension, followed by an assessment of the solution composition, from which the solution concentration can be determined.
How do you calculate dissolved mass in a solution?
Rearranging the equation for concentration allows the mass of solute to be calculated:mass of solute in g = concentration in g/dm 3 × volume in dm 3A solution of sodium chloride has a concentration of 10 g/dm 3. ... mass of solute in g = concentration in g/dm 3 × volume in dm 3= 10 g/dm 3 × 2 dm 3
How do you calculate the amount of solute dissolved?
This percentage can be determined in one of three ways: (1) the mass of the solute divided by the mass of solution, (2) the volume of the solute divided by the volume of the solution, or (3) the mass of the solute divided by the volume of the solution.
How do you solve solubility problems?
0:315:30Solubility Problems - YouTubeYouTubeStart of suggested clipEnd of suggested clipIf the number is equal to or greater than the solubility that we're going to get then it must beMoreIf the number is equal to or greater than the solubility that we're going to get then it must be saturated. And if it's less than this the solubility then it's going to be unsaturated.
How do you calculate solubility GCSE?
Determining solubilityMeasure accurately 100 cm 3 of water and add to a beaker.Add small amounts of the solute until no more can dissolve.Record the mass of an evaporating dish.Filter the mixture so the undissolved solid is left behind and the solution is in the evaporating dish.More items...
How do you calculate solubility from KSP?
0:152:55How to Calculate Solubility from Solubility Product - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo my ksp will be a g plus concentration which is x b r minus concentration which is also x soMoreSo my ksp will be a g plus concentration which is x b r minus concentration which is also x so therefore this would be an x squared.
How do you calculate solubility in a lab?
2:323:31Lab 13.2 - Determining Solubility - YouTubeYouTubeStart of suggested clipEnd of suggested clipFor example if we explode some salt while scooping it into the test tube our data would not beMoreFor example if we explode some salt while scooping it into the test tube our data would not be accurate or if we kept scooping salt into the solvent before the solute.
How do you measure solubility in an experiment?
Add a small amount of the solute to the water and stir with a clean disposable spoon until dissolved. Repeat this process, always adding a small amount until the solute will no longer dissolve. Weigh the amount of solute remaining to determine how much was added to the solution.
What is unit of solubility?
The unit of solubility is generally in mg/L (milligrams per liter) or ppm (parts per million).
How do you calculate the number of grams?
0:201:15How to Calculate Grams from Moles (Moles to Mass) - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe molar mass of oxygen is 32 grams per mole and we have ten point two moles of it. MultiplyingMoreThe molar mass of oxygen is 32 grams per mole and we have ten point two moles of it. Multiplying those two together we get three hundred twenty six point four grams.
How many grams of KNO3 will dissolve in 100g of water at 30 C?
10gAt 30°C approximately 10g of KCIO3 will dissolve in 100g of water. If the temperature is increased to 80°C, approximately 40g of the substance will dissolve in 100g (or 100mL) of water. Directions: Use the graph to answer the following questions. REMEMBER UNITS!
How do you find grams of solute using Molality?
0:191:34Calculating moles and grams of solute from molality - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo to figure out the grams we have to figure out the moles of the solute. First. So that is going toMoreSo to figure out the grams we have to figure out the moles of the solute. First. So that is going to be given to us by multiplying molality times the kilograms of solvent.
What is 5 grams of sugar dissolved in 25 grams of solvent?
Answer. Explanation: The mass by volume percentage of 250 ml of a solution containing 5 g of sugar is 2%.
How to calculate grams per liter?
1) multiply the g/100mL value by 10/10 . This converts it to grams per 1000 mL or, better yet, grams per liter. (Sometimes the data is given in g/L. When that happens, this step is skipped.) 2) divide the grams per liter value by the molar mass of the substance. This gives moles per liter, which is molar solubility.
How many grams of salt are in a mL of Cu(IO4)2?
Example #6:300. mL of a saturated solution of Cu(IO4)2contains 0.380 grams of dissolved salt. Determine the Ksp.
How much calcium arsenate is solubility?
I used the Wikipedia entry for calcium arsenate,where the solubility is given as 0.013 g/100mL.
How many 250ml in 1 liter?
Comment: there are four 250mL amounts in 1 liter
How Do You Find Solubility?
Generally this is done by introducing a gram (or other measured amount) of the chemical to solvent one part at a time. Another part is added until the solute has been completely dissolved. The amount of parts that needed to be added for complete dissolution is the number of parts that the solute requires to create a saturated solution. Once you know how many parts of solvent and how many parts of solute you need, you can then look at the general solubility descriptions (outlined below). Solubility should almost always be tested at room temperature, as temperature greatly impacts the results.
What is the solubility of a chemical?
The solubility of a chemical is its ability to dissolve when presented with a certain solvent. When it comes to chemicals, it's often important to know how soluble the product is and which solvents are most appropriate for its applications.
What Is Chemical Solubility?
Chemical solubility is a very specific measurement: the maximum amount of the chemical that can be dissolved in a given solvent. Once the solvent has been introduced to as much of the chemical as it possibly can be without beginning to "reject" the chemical, the resulting solution is known as "saturated." A fully saturated solution gives you the solubility of the chemical, but note that this is affected by the solvent as well. It is possible to introduce more of a chemical beyond a certain saturation point, but the resulting mixture will be chemically unstable and will "want" to reject the excess solute.
Why is solubility important?
Solubility is very important for those working with chemicals and solutions of all types. Though solubility information should be readily available for most chemicals and compounds, it can sometimes be necessary to either test or verify the chemical's saturation point independently.
Why is it important to determine the solubility of a chemical?
It's often necessary to determine the solubility of a chemical before you can begin working with it. Chemicals, especially those used in labs, are often concentrated and distilled, in order to be easier to use, transport, and store. But these highly concentrated chemicals will have to be properly reconstituted if they are to be used reliably.
How many parts of solvent are needed for a single part of a solution?
Here are the terms that you should know. Insoluble. If the chemical requires more than 10,000 parts of solvent for a single part of solute.
How much solvent is needed for a solute?
If the chemical requires 30 to 100 parts of solvent for a single part of solute. Soluble. If the chemical requires 10 to 30 parts of solvent for a single part of solute. Freely soluble. If the chemical requires 1 to 10 parts of solvent for a single part of solute. Very soluble. If the chemical requires less than 1 part of solvent ...