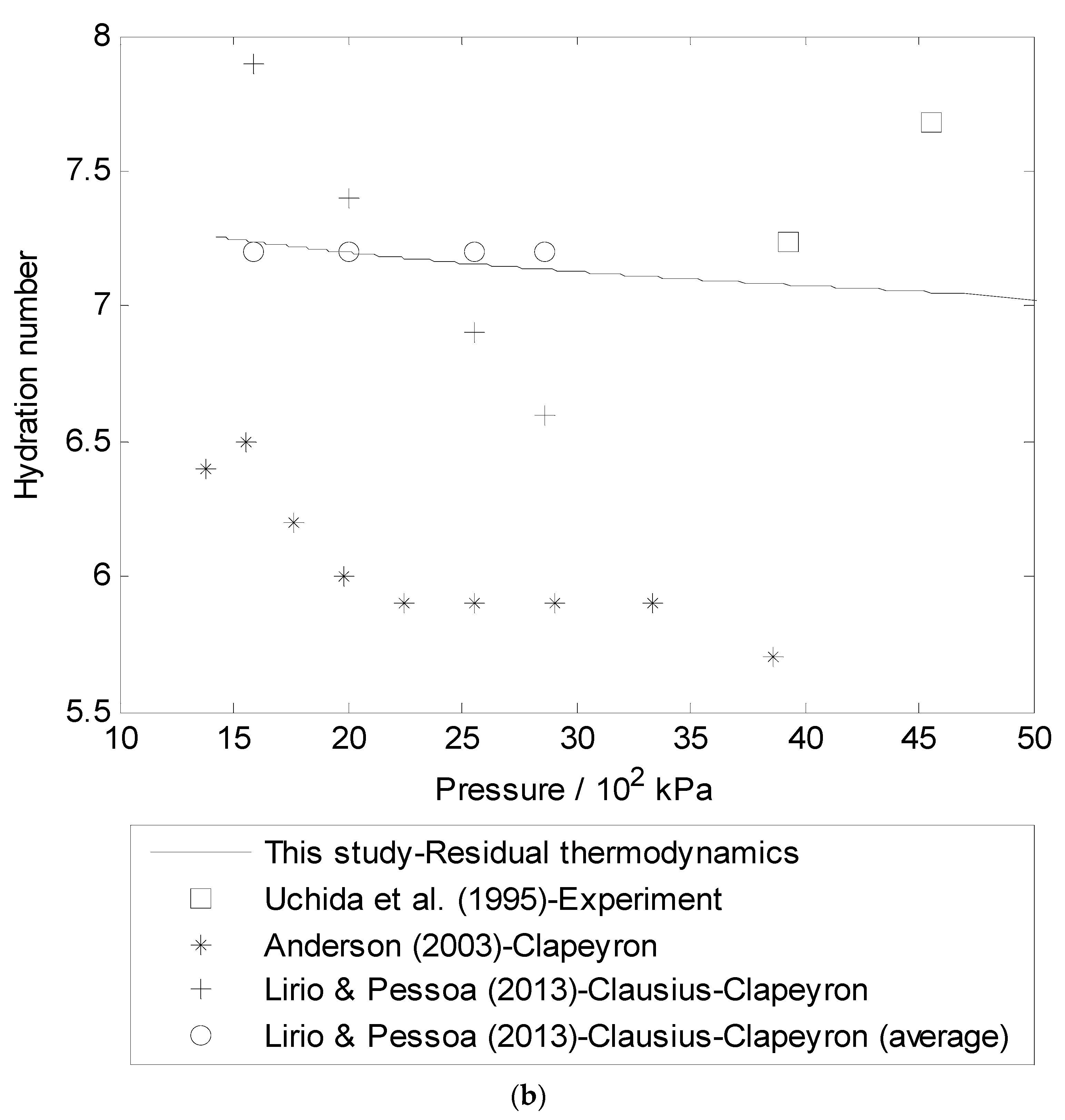
The heat of hydration formula is given by: Heat of hydration = ΔH solution – ΔH lattice energy. Where, ΔH solution = Heat of the solution. ΔH lattice energy = Lattice energy of the solution.
What is the formula for heat of hydration?
The heat of hydration formula is given by: Heat of hydration = (ΔH solution – ΔH lattice energy) Where. ΔH solution = Heat of the solution. ΔH lattice energy = Lattice energy of the solution. Example 1. The sodium chloride lattice enthalpy is ΔH for NaCl →→ Na+ + Cl– is 700 kJ/mol.
How do you calculate the heat of hydration in cement?
Heat evolved by chemical reactions with water, such as that evolved during the setting and hardening of Portland cement, or the difference between the heat of solution of dry cement and that of partially hydrated cement Formula to Determine Heat of Hydration H = H1 – H2 – 0.4 (th – 25.0)
How do you determine the heat of hydration of salt?
The heat of hydration can be determined if the heat of the solutions of anhydrous salt and the hydrated forms are known. Anhydrous salts readily combine with water to form hydrates and dissolve with the evolution of heat. The only difference between hydrate and anhydrous salt is the heat is evolved as heat...
How to calculate the specific heat of a partially hydrated sample?
Thus, for the partially hydrated sample, which has a specific heat of approximately 1.7 kJ/kg (0.4 cal/g) of ignited cement, if the final calorimeter temperature exceeds the temperature of the sample at the time it was introduced, add a correction of 1.7 kJ/kg·K (°C) difference in those temperatures when calculating the heat of solution.
What is the formula of heat of hydration?
Anhydrous salts readily combine with water to form hydrates and dissolve with the evolution of heat. The only difference between hydrate and anhydrous salt is the heat is evolved as the heat of hydration in the formation of hydrates. The sodium chloride lattice enthalpy is ΔH for NaCl →→ Na+ + Cl– is 700 kJ/mol.
How do you calculate the heat of hydration of concrete?
The heat of hydration of the cement was calculated to the nearest kilojoule, as follows(13) H = H 1 − H 2 − 0.4 ( t h − 25 ) Where: H = heat of hydration of ignited cement, kJ/kg. H1 = heat of solution of dry cement. H2 = heat of solution of partially hydrated sample.
How do you calculate the hydration enthalpy of a solution?
0:086:4515.1 Enthalpy change of solution and hydration (HL) - YouTubeYouTubeStart of suggested clipEnd of suggested clipProcess the dissolving of an ionic compound can be divided into two steps in the first step theMoreProcess the dissolving of an ionic compound can be divided into two steps in the first step the solid ionic compound is broken down into gaseous ions.
How do you measure the degree of hydration?
The degree of hydration is therefore determined by weighing the sample after drying to +105 ºC and after ignition to 1000 ºC. The weight difference corresponds to the chemically bound water content.
What is hydration and heat of hydration of cement?
The chemical reaction that takes place between the cement and water is referred to as hydration of the cement. The hydration reaction is an exothermic reaction. The cement hydration will liberate a considerable amount of heat. This is called as Heat of liberation or Heat of Hydration.
What is the meaning of 1 2 4 in concrete?
The specification should also say if those proportions are by weight or volume. In mix design the proportion 1: 2:4 means, 1 part of cement is mixed with 2 part of sand and 4 part of coarse aggregate.
How do you calculate heat of solution?
Heat of Solution or Enthalpy of Solution Chemistry TutorialStep 1: Calculate the amount of energy released or absorbed (q) q = m × Cg × ΔT. ... Step 2: Calculate moles of solute (n) n = m ÷ M. ... Step 3: Calculate mount of energy (heat) released or absorbed per mole of solute (ΔHsoln) ΔHsoln = q ÷ n.
What formula is Q MC ∆ T?
The amount of heat gained or lost by a sample (q) can be calculated using the equation q = mcΔT, where m is the mass of the sample, c is the specific heat, and ΔT is the temperature change.
What do you mean by hydration enthalpy?
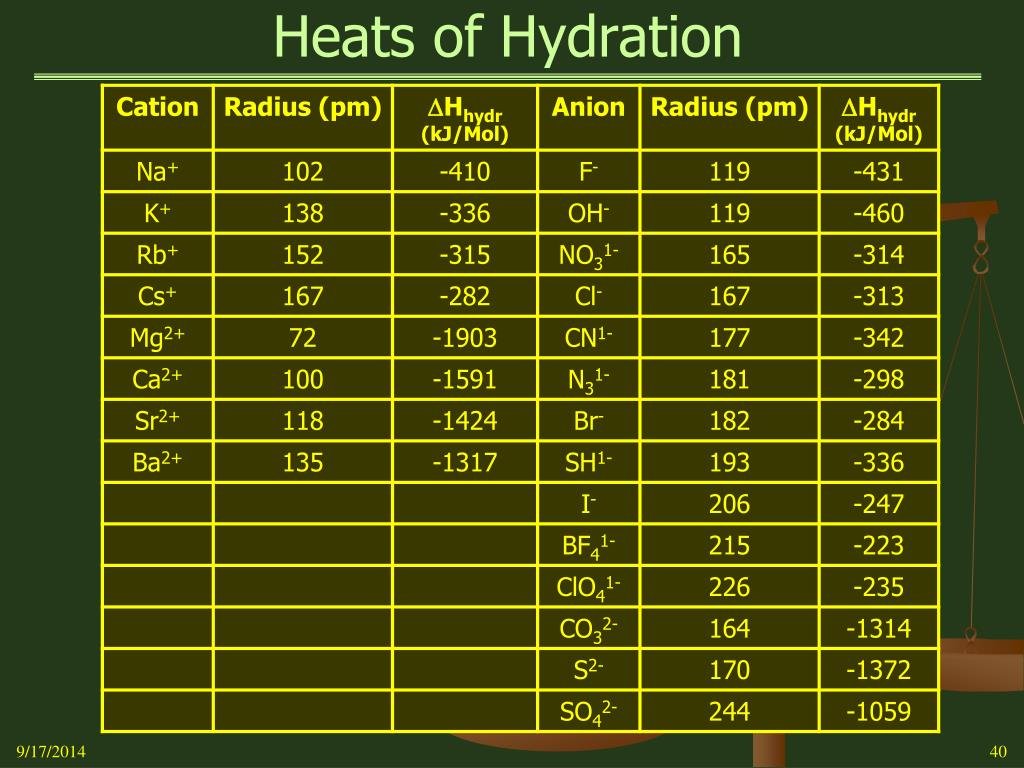
The heat energy released when new bonds are made between the ions and water molecules is known as the hydration enthalpy of the ion. The hydration enthalpy is the enthalpy change when 1 mole of gaseous ions dissolve in sufficient water to give an infinitely dilute solution. Hydration enthalpies are always negative.
What does heat of hydration depend on?
The amount of heat released depends on the cement composition, curing temperature, water to cement ratio, and cement fineness.
Which has greatest degree of hydration?
Li+ ionLi+ ion has maximum degree of hydration.
Which alkali metal has the highest hydration enthalpy and why?
Since size of atoms increases down the group in alkali metals and charge remains the same Hydration enthalpy decreases down the group which means Lithium have highest hydration enthalpy and Caesium have lowest hydration enthalpy among alkali metals.
How much is the heat of hydration of cement?
The heat of hydration 420 J/g.
What is rate of hydration of cement?
The reaction of compound C3A with water is very fast and is responsible for flash setting of cement (stiffening without strength development) and thus it will prevent the hydration of C3S and C2S.
Why is heat of hydration of cement important?
The rate of heat generation during cement hydration increases with temperature and at low temperature hydration reaction can become extremely slow. Indeed the temperature in the annulus is one of the most important factors influencing the development of the cement compressive strength.
Is heat of hydration always negative?
The hydration enthalpy is the enthalpy change when 1 mole of gaseous ions dissolve in sufficient water to give an infinitely dilute solution. Hydration enthalpies are always negative.
What is Heat of Hydration?
The reaction of cement with water is exothermic which liberates a considerable quantity of heat, This liberation of heat is called heat of hydration. The heat is delivered by hydration of one mole of particles at a consistent pressure. The more the particle is hydrated, the more heat is delivered.
Formula for Heat of Hydration
The Heat of Hydration formula is characterized by how much energy is delivered when one mole of particles goes through hydration. The heat of hydration recipe is given by,
What is the heat of hydration?from byjus.com
The heat of hydration is described as the quantity of energy produced when one mole of ions undergo a hydration process. It is a specific form of dissolution energy and the solvent used is water.
What is the difference between anhydrous salt and hydrate?from byjus.com
The only difference between hydrate and anhydrous salt is the heat is evolved as the heat of hydration in the formation of hydrates. The heat of hydration formula is given by:
What temperature can you heat a concrete slab?from civilmedium.com
Heating the concrete more than 158 °F (70 °C) can lead to delayed ettringite formation and ultimately cracks the concrete. Therefore the cracking possibility of concrete due to heat of hydration should be closely analyzed well before the placement and reduce the risk as much as possible.
How many steps are required for thermal analysis of concrete?from civilmedium.com
A thermal analysis of concrete follows in 10 steps. All the inputs are summarized below.
How many degrees of temperature difference between internal and surface temperatures of concrete?from civilmedium.com
Users can try different starting times and placing temperatures to get the least peak temperature as well as the least temperature different. More than 19 degrees of the temperature difference between internal and surface temperatures of concrete is considered as critical by the program.
What is the maximum temperature difference?from civilmedium.com
The maximum temperature difference is safe to maintain below 35 °F (20°C) degrees. Users can try different starting times and placing temperatures to get the least peak temperature as well as the least temperature different.
How to reduce peak temperature of concrete?from civilmedium.com
By adjusting the starting time of the concrete, we can alter the peak temperature. This results in reducing the temperature difference too. The program allows you to try out different placing times as well as days for the analysis and select a suitable time for the work. The above example uses 7 am as the starting time of the concrete. The following results show the peak heat difference in case if the same work started at 7 pm.
What Is Heat Of Hydration?
The heat of hydration is a reaction of cement with water that is exothermic, releasing a significant amount of heat. The heat release is further termed as the heat of hydration.
Formula For Heat Of Hydration
The heat of hydration formula describes how much energy is delivered when one mole of particles is hydrated. The recipe for the heat of hydration is provided by,
Hydration Reaction
When hydrogen and hydroxyl ions are bound to a carbon in a carbon double bond, a hydration process occurs. Most of the time, one reactant (usually an alkene or an alkyne) reacts with water to generate ethanol, isopropanol, or 2-butanol (alcohols).
Heat Of Hydration In Cement
When concrete is mixed with water, an exothermic reaction occurs, resulting in a large amount of heat being released. At lower temperatures, it may take a longer duration of the day to establish a concrete hydration reaction. Rising temperatures cause a rapid creation of heat.
Things To Remember
A mole whose hydration is particularly developed is an example of the heat of hydration.
Why do we need hydration calculator?from gigacalculator.com
Benefits of staying optimally hydrated. One of the reasons to use a hydration calculator is to maintain a healthy life, but scientific studies also link adequate water intake to benefits for the treatment of health conditions as well as mental state improvement.
How do you know if you are well hydrated?from slenderkitchen.com
The easiest way to know that you are well hydrated is to pay attention to your urine. Generally speaking, it should be light yellow or clear without too much of a smell. Thirst is another indicator, but many people confuse this with hunger.
What other questions do you have about how much water to drink?from slenderkitchen.com
The content of this field is kept private and will not be shown publicly.
What fluid intake charts contain?from printablee.com
In order to know the fluid intake that you do every day, you better use the fluid intake charts. This chart consists of the amount of fluid that you have consumed. The easiest way, make a cup of water image that matches the capacity of the glass you normally use. In one day, make sure the cup is in accordance with the normal amount of fluid that should be consumed by people. You can make it like a checklist. If you don't meet the fluid intake on the chart every day, an effort will be made to make the chart useful.
Does Coke count as water intake?from printablee.com
Fluid intake needed by the body is not only water but also other types that have a fluid form. Coke, coffee, tea and other drinks can be included in the fluid intake category. It's just recommended to consume water because it is not mixed with other ingredients that have flavourings. You can still consume juice, coke and others. But it would be better dominated by water content to achieve a healthy body without a mixture of sugar and other substances present in coke, juice, caffeine and tea.
How does dehydration affect athletes?from gigacalculator.com
During challenging athletic events, it is not uncommon for athletes to lose 6–10% of body weight in sweat loss, thus leading to dehydration if fluids have not been adequately replenished. Decrements in physical performance in athletes have been observed under much lower levels of dehydration: as little as 2% Under relatively mild levels of dehydration, individuals engaging in rigorous physical activity will experience decrements in performance related to reduced endurance, increased fatigue, altered thermoregulatory capability, reduced motivation, and increased perceived effort. Rehydration can reverse these deficits, and also reduce oxidative stress induced by exercise and dehydration. Hypohydration appears to have a more significant impact on high-intensity and endurance activity such as tennis and long-distance running than on anaerobic activities such as weight lifting or on shorter-duration activities, such as rowing [1].
How much fluid should a man drink a day?from printablee.com
Because men and women have different activities, normal standards for fluid intake are also different. For men, they should consume 3.7 litres of fluid in one day, while for women, only 1 litre is less than men, ...
How to Calculate Dough Hydration
Calculating dough hydration is the only way to know exactly what percentage of hydration your bread dough will end up with. With the statistics, you can learn how many crumbs your bread will have (more on this later – stay tuned! ).
How to Calculate Dough Hydration – Backwards
Calculate hydration backward? But why, Michelle? No, I’m not trying to be snazzy. Some bakers want a specific percentage of hydration. In this case, they’ll need to find out how much water to add to their flour.
Why is Calculating Dough Hydration Important?
Baking bread is already challenging enough. The kneading, proofing, and shaping – yeah, it’s a process. Why would you want to dedicate even more time to baking bread by calculating the dough’s hydration? What’s the purpose behind it?
FAQs
Calculating your dough hydration can seem excessive, but it’s the gateway to perfect results every time – and it’s not that difficult or time-consuming! You’ve already learned a lot, but I thought I’d add a few more frequently asked questions to help you out.
Calculating Dough Hydration is Easy and Makes Incredible Bread!
Calculating dough hydration is a simple three-step process. Simply divide the weight of the water (in grams) by the weight of the flour (also in grams). Then, multiply it by 100. You will have your percentage and know precisely what type of crumb your bread will end up with.